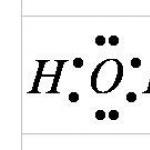Water is a familiar and unusual substance. Almost 3/4 of the surface of our planet is occupied by oceans and seas. Hard water - snow and ice - covers 20% of the land. The climate of the planet depends on water. Geophysicists say that The earth would have cooled long ago and turned into a lifeless piece of stone, if not for the water. It has a very high heat capacity. When heated, it absorbs heat; cooling down, he gives it away. Earth's water both absorbs and returns a lot of heat and thereby “evens out” the climate. And what protects the Earth from cosmic cold are those water molecules that are scattered in the atmosphere - in clouds and in the form of vapor.
Water is the most mysterious substance in nature after DNA, possessing unique properties that not only have not yet been fully explained, but are far from all known. The longer it is studied, the more new anomalies and mysteries are found in it. Most of these anomalies that make life possible on Earth are explained by the presence of hydrogen bonds between water molecules, which are much stronger than the van der Waals forces of attraction between molecules of other substances, but an order of magnitude weaker than ionic and covalent bonds between atoms in molecules. The same hydrogen bonds are also present in the DNA molecule.
A water molecule (H 2 16 O) consists of two hydrogen atoms (H) and one oxygen atom (16 O). It turns out that almost the entire variety of properties of water and the unusualness of their manifestation are determined, ultimately, by the physical nature of these atoms, the way they are combined into a molecule and the grouping of the resulting molecules.
Rice. Structure of a water molecule . Geometric diagram (a), flat model (b) and spatial electronic structure (c) of the H2O monomer. Two of the four electrons in the outer shell of the oxygen atom are involved in creating covalent bonds with hydrogen atoms, and the other two form highly elongated electron orbits, the plane of which is perpendicular to the H-O-H plane.
The water molecule H 2 O is built in the form of a triangle: the angle between the two oxygen-hydrogen bonds is 104 degrees. But since both hydrogen atoms are located on the same side of the oxygen, the electrical charges in it are dispersed. The water molecule is polar, which is the reason for the special interaction between its different molecules. The hydrogen atoms in the H 2 O molecule, having a partial positive charge, interact with the electrons of the oxygen atoms of neighboring molecules. This chemical bond is called a hydrogen bond. It unites H 2 O molecules into unique associates of spatial structure; the plane in which the hydrogen bonds are located is perpendicular to the plane of the atoms of the same H 2 O molecule. The interaction between water molecules primarily explains the abnormally high temperatures of its melting and boiling. Additional energy must be supplied to loosen and then destroy hydrogen bonds. And this energy is very significant. This is why the heat capacity of water is so high.
 A water molecule contains two polar covalent bonds H–O. They are formed due to the overlap of two one-electron p - clouds of an oxygen atom and one-electron S - clouds of two hydrogen atoms.
A water molecule contains two polar covalent bonds H–O. They are formed due to the overlap of two one-electron p - clouds of an oxygen atom and one-electron S - clouds of two hydrogen atoms.
In accordance with the electronic structure of hydrogen and oxygen atoms, a water molecule has four electron pairs. Two of them are involved in the formation of covalent bonds with two hydrogen atoms, i.e. are binding. The other two electron pairs are free - non-bonding. They form an electron cloud. The cloud is heterogeneous - individual concentrations and rarefactions can be distinguished in it.
A water molecule has four pole charges: two positive and two negative. Positive charges are concentrated on hydrogen atoms, since oxygen is more electronegative than hydrogen. The two negative poles come from two non-bonding electron pairs of oxygen.
An excess electron density is created at the oxygen core. The internal electron pair of oxygen evenly frames the nucleus: schematically it is represented by a circle with the center - the O 2- nucleus. The four outer electrons are grouped into two electron pairs that gravitate towards the nucleus, but are partially not compensated. Schematically, the total electron orbitals of these pairs are shown in the form of ellipses elongated from a common center - the O 2- nucleus. Each of the remaining two electrons in oxygen pairs with one electron in hydrogen. These vapors also gravitate towards the oxygen core. Therefore, hydrogen nuclei - protons - turn out to be somewhat bare, and a lack of electron density is observed here.
 Thus, in a water molecule there are four charge poles: two negative (excess electron density in the region of the oxygen nucleus) and two positive (lack of electron density in the two hydrogen nuclei). For greater clarity, we can imagine that the poles occupy the vertices of a deformed tetrahedron, in the center of which there is an oxygen nucleus.
Thus, in a water molecule there are four charge poles: two negative (excess electron density in the region of the oxygen nucleus) and two positive (lack of electron density in the two hydrogen nuclei). For greater clarity, we can imagine that the poles occupy the vertices of a deformed tetrahedron, in the center of which there is an oxygen nucleus.
Rice. Structure of a water molecule: a – angle between O-H bonds; b – location of charge poles; c – appearance of the electron cloud of a water molecule.
The almost spherical water molecule has a noticeably pronounced polarity, since the electrical charges in it are located asymmetrically. Each water molecule is a miniature dipole with a high dipole moment of 1.87 deBy. Debye is an off-system unit of electric dipole 3.33564·10 30 C·m. Under the influence of water dipoles, interatomic or intermolecular forces on the surface of a substance immersed in it are weakened by 80 times. In other words, water has a high dielectric constant, the highest of all compounds known to us.
Largely due to this, water manifests itself as a universal solvent. Solids, liquids, and gases are subject to its dissolving action to one degree or another.
The specific heat capacity of water is the highest of all substances. In addition, it is 2 times higher than that of ice, while for most simple substances (for example, metals) the heat capacity practically does not change during the melting process, and for substances made of polyatomic molecules it, as a rule, decreases during melting.
Such an understanding of the structure of the molecule makes it possible to explain many properties of water, in particular the structure of ice. In the ice crystal lattice, each molecule is surrounded by four others. In a planar image, this can be represented as follows:

The connection between molecules is carried out through a hydrogen atom. The positively charged hydrogen atom of one water molecule is attracted to the negatively charged oxygen atom of another water molecule. This bond is called a hydrogen bond (it is designated by dots). The strength of a hydrogen bond is approximately 15-20 times weaker than a covalent bond. Therefore, the hydrogen bond is easily broken, which is observed, for example, during the evaporation of water.
 Rice. left - Hydrogen bonds between water molecules
Rice. left - Hydrogen bonds between water molecules
The structure of liquid water resembles that of ice. In liquid water, molecules are also connected to each other through hydrogen bonds, but the structure of water is less “rigid” than that of ice. Due to the thermal movement of molecules in water, some hydrogen bonds are broken and others are formed.

Rice. Crystal lattice of ice. The water molecules H 2 O (black balls) in its nodes are located so that each has four “neighbors”.
The polarity of water molecules and the presence of partially uncompensated electrical charges in them gives rise to a tendency to group molecules into large “communities” - associates. It turns out that only water in a vapor state fully corresponds to the formula H2O. This was shown by the results of determining the molecular mass of water vapor. In the temperature range from 0 to 100°C, the concentration of individual (monomeric molecules) of liquid water does not exceed 1%. All other water molecules are combined into associates of varying degrees of complexity, and their composition is described by the general formula (H 2 O)x.
The direct cause of the formation of associates is hydrogen bonds between water molecules. They arise between the hydrogen nuclei of some molecules and the electron “condensations” of the oxygen nuclei of other water molecules. True, these bonds are tens of times weaker than “standard” intramolecular chemical bonds, and ordinary molecular movements are enough to destroy them. But under the influence of thermal vibrations, new connections of this type just as easily arise. The emergence and decay of associates can be expressed by the following diagram:
x·H 2 O↔ (H 2 O) x
Since the electron orbitals in each water molecule form a tetrahedral structure, hydrogen bonds can arrange the arrangement of water molecules into tetrahedral coordinated associates.
Most researchers explain the anomalously high heat capacity of liquid water by the fact that when ice melts, its crystalline structure does not immediately collapse. In liquid water, hydrogen bonds between molecules are preserved. What remains in it are fragments of ice - associates of a large or smaller number of water molecules. However, unlike ice, each associate does not exist for long. The destruction of some and the formation of other associates constantly occurs. At each temperature value in water, its own dynamic equilibrium is established in this process. And when water is heated, part of the heat is spent on breaking hydrogen bonds in associates. In this case, 0.26-0.5 eV is spent on breaking each bond. This explains the anomalously high heat capacity of water compared to melts of other substances that do not form hydrogen bonds. When heating such melts, energy is spent only on imparting thermal movements to their atoms or molecules. Hydrogen bonds between water molecules are completely broken only when water turns into steam. The correctness of this point of view is also indicated by the fact that the specific heat capacity of water vapor at 100°C practically coincides with the specific heat capacity of ice at 0°C.
Picture below:
The elementary structural element of an associate is a cluster: Rice. A separate hypothetical water cluster. Individual clusters form associates of water molecules (H 2 O) x: Rice. Clusters of water molecules form associates.

There is another point of view on the nature of the anomalously high heat capacity of water. Professor G.N. Zatsepina noted that the molar heat capacity of water, amounting to 18 cal/(molgrad), is exactly equal to the theoretical molar heat capacity of a solid with triatomic crystals. And in accordance with the law of Dulong and Petit, the atomic heat capacities of all chemically simple (monatomic) crystalline bodies at a sufficiently high temperature are the same and equal to 6 calDmol o deg). And for triatomic ones, the grammol of which contains 3 N a crystal lattice sites, it is 3 times more. (Here N a is Avogadro’s number).
It follows that water is, as it were, a crystalline body consisting of triatomic H 2 0 molecules. This corresponds to the common idea of water as a mixture of crystal-like associates with a small admixture of free H 2 O water molecules between them, the number of which increases with increasing temperature. From this point of view, what is surprising is not the high heat capacity of liquid water, but the low heat capacity of solid ice. The decrease in the specific heat capacity of water during freezing is explained by the absence of transverse thermal vibrations of atoms in the rigid crystal lattice of ice, where each proton that causes a hydrogen bond has only one degree of freedom for thermal vibrations instead of three.
But due to what and how can such large changes in the heat capacity of water occur without corresponding changes in pressure? To answer this question, let's meet with the hypothesis of the candidate of geological and mineralogical sciences Yu. A. Kolyasnikov about the structure of water.
He points out that the discoverers of hydrogen bonds, J. Bernal and R. Fowler, in 1932 compared the structure of liquid water with the crystalline structure of quartz, and those associates mentioned above are mainly 4H 2 0 tetramers, in which there are four molecules waters are connected into a compact tetrahedron with twelve internal hydrogen bonds. As a result, a tetrahedron is formed.
At the same time, hydrogen bonds in these tetramers can form both right-handed and left-handed sequences, just as crystals of widespread quartz (Si0 2), which also have a tetrahedral structure, come in right- and left-handed rotational crystal forms. Since each such water tetramer also has four unused external hydrogen bonds (like one water molecule), the tetramers can be connected by these external bonds into a kind of polymer chains, like a DNA molecule. And since there are only four external bonds, and 3 times more internal ones, this allows heavy and strong tetramers in liquid water to bend, turn and even break these external hydrogen bonds weakened by thermal vibrations. This determines the fluidity of water.
Water, according to Kolyasnikov, has this structure only in the liquid state and, possibly, partially in the vapor state. But in ice, the crystal structure of which has been well studied, tetrahydrols are connected to each other by inflexible, equally strong direct hydrogen bonds into an openwork frame with large voids in it, which makes the density of ice less than the density of water.
 Rice. Crystal structure of ice: water molecules are connected in regular hexagons
Rice. Crystal structure of ice: water molecules are connected in regular hexagons
When ice melts, some of the hydrogen bonds in it weaken and bend, which leads to a restructuring of the structure into the above-described tetramers and makes liquid water more dense than ice. At 4°C, a state occurs when all hydrogen bonds between tetramers are maximally bent, which determines the maximum density of water at this temperature. There is nowhere for connections to go any further.
At temperatures above 4°C, individual bonds between tetramers begin to break, and at 36-37°C, half of the external hydrogen bonds are broken. This determines the minimum on the curve of the specific heat capacity of water versus temperature. At a temperature of 70°C, almost all intertetramer bonds are broken, and along with free tetramers, only short fragments of “polymer” chains of them remain in water. Finally, when water boils, the final rupture of now single tetramers into individual H 2 0 molecules occurs. And the fact that the specific heat of evaporation of water is exactly 3 times greater than the sum of the specific heats of melting ice and subsequent heating of water to 100 ° C confirms Kolyasnikov’s assumption About. that the number of internal bonds in a tetramer is 3 times greater than the number of external ones.
This tetrahedral-helical structure of water may be due to its ancient rheological connection with quartz and other silicon-oxygen minerals that predominate in the earth's crust, from the depths of which water once appeared on Earth. Just as a small crystal of salt causes the solution surrounding it to crystallize into similar crystals, and not into others, so quartz caused water molecules to line up in tetrahedral structures, which are energetically most favorable. And in our era, in the earth’s atmosphere, water vapor, condensing into droplets, forms such a structure because the atmosphere always contains tiny droplets of aerosol water that already have this structure. They are centers of condensation of water vapor in the atmosphere. Below are possible chain silicate structures based on the tetrahedron, which can also be composed of water tetrahedra.
Rice. Elementary regular silicon-oxygen tetrahedron SiO 4 4-.
Rice. Elementary silicon-oxygen units-orthogroups SiO 4 4- in the structure of Mg-pyroxene enstatite (a) and diortho groups Si 2 O 7 6- in the Ca-pyroxenoid wollastonite (b).

Rice. The simplest types of island silicon-oxygen anionic groups: a-SiO 4, b-Si 2 O 7, c-Si 3 O 9, d-Si 4 O 12, d-Si 6 O 18.

Rice. below - The most important types of silicon-oxygen chain anionic groups (according to Belov): a-metagermanate, b - pyroxene, c - bathysite, d-wollastonite, d-vlasovite, e-melilite, f-rhodonite, z-pyroxmangite, i-metaphosphate, k - fluoroberyllate, l - barylite.

Rice. below - Condensation of pyroxene silicon-oxygen anions into honeycomb two-row amphibole (a), three-row amphibole-like (b), layered talc and related anions (c).

Rice. below - The most important types of banded silicon-oxygen groups (according to Belov): a - sillimanite, amphibole, xonotlite; b-epididymitis; β-orthoclase; g-narsarsukite; d-phenacite prismatic; e-euclase inlaid.

 Rice. on the right - A fragment (elementary package) of the layered crystal structure of muscovite KAl 2 (AlSi 3 O 10 XOH) 2, illustrating the interlayering of aluminum-silicon-oxygen networks with polyhedral layers of large aluminum and potassium cations, reminiscent of a DNA chain.
Rice. on the right - A fragment (elementary package) of the layered crystal structure of muscovite KAl 2 (AlSi 3 O 10 XOH) 2, illustrating the interlayering of aluminum-silicon-oxygen networks with polyhedral layers of large aluminum and potassium cations, reminiscent of a DNA chain.
Other models of water structure are also possible. Tetrahedrally bound water molecules form peculiar chains of fairly stable composition. Researchers are uncovering increasingly subtle and complex mechanisms of the “internal organization” of the water mass. In addition to the ice-like structure, liquid water and monomer molecules, a third element of the structure is also described - non-tetrahedral.
A certain part of water molecules are associated not in three-dimensional frameworks, but in linear ring associations. The rings, when grouped, form even more complex complexes of associates.
Thus, water can theoretically form chains, like a DNA molecule, as will be discussed below. Another interesting thing about this hypothesis is that it implies the equal probability of the existence of right- and left-handed water. But biologists have long noticed that in biological tissues and structures only either left- or right-handed formations are observed. An example of this is protein molecules, built only from left-handed amino acids and twisted only in a left-handed spiral. But sugars in nature are all right-handed. No one has yet been able to explain why in living nature there is such a preference for the left in some cases and for the right in others. Indeed, in inanimate nature, both right-handed and left-handed molecules are found with equal probability.
More than a hundred years ago, the famous French naturalist Louis Pasteur discovered that organic compounds in plants and animals are optically asymmetrical - they rotate the plane of polarization of the light incident on them. All amino acids that make up animals and plants rotate the plane of polarization to the left, and all sugars rotate to the right. If we synthesize compounds with the same chemical composition, then each of them will contain an equal number of left- and right-handed molecules.
As you know, all living organisms consist of proteins, and they, in turn, are made of amino acids. By combining with each other in a variety of sequences, amino acids form long peptide chains that spontaneously “twist” into complex protein molecules. Like many other organic compounds, amino acids have chiral symmetry (from the Greek chiros - hand), that is, they can exist in two mirror-symmetrical forms called “enantiomers”. Such molecules are similar to one another, like the left and right hands, so they are called D- and L-molecules (from the Latin dexter, laevus - right and left).
 Now let us imagine that a medium with left and right molecules has passed into a state with only left or only right molecules. Experts call such an environment chirally (from the Greek word “cheira” - hand) ordered. Self-reproduction of living things (biopoiesis - as defined by D. Bernal) could arise and be maintained only in such an environment.
Now let us imagine that a medium with left and right molecules has passed into a state with only left or only right molecules. Experts call such an environment chirally (from the Greek word “cheira” - hand) ordered. Self-reproduction of living things (biopoiesis - as defined by D. Bernal) could arise and be maintained only in such an environment.
Rice. Mirror symmetry in nature
Another name for enantiomer molecules - "dextrorotatory" and "levorotatory" - comes from their ability to rotate the plane of polarization of light in different directions. If linearly polarized light is passed through a solution of such molecules, the plane of its polarization rotates: clockwise if the molecules in the solution are right-handed, and counterclockwise if the molecules in the solution are left-handed. And in a mixture of equal amounts of D- and L-forms (called a “racemate”), the light will retain its original linear polarization. This optical property of chiral molecules was first discovered by Louis Pasteur in 1848.
It is curious that almost all natural proteins consist only of left-handed amino acids. This fact is all the more surprising since the synthesis of amino acids in laboratory conditions produces approximately the same number of right- and left-handed molecules. It turns out that not only amino acids have this feature, but also many other substances important for living systems, and each has a strictly defined sign of mirror symmetry throughout the biosphere. For example, sugars that are part of many nucleotides, as well as nucleic acids DNA and RNA, are represented in the body exclusively by right-handed D-molecules. Although the physical and chemical properties of the “mirror antipodes” are the same, their physiological activity in organisms is different: L-caxara are not absorbed, L-phenylalanine, unlike its harmless D-molecules, causes mental illness, etc.
According to modern ideas about the origin of life on Earth, the choice of a certain type of mirror symmetry by organic molecules served as the main prerequisite for their survival and subsequent self-reproduction. However, the question of how and why the evolutionary selection of one or another mirror antipode occurred still remains one of the biggest mysteries of science.
The Soviet scientist L.L. Morozov proved that the transition to chiral order could not occur evolutionarily, but only with some specific sharp phase change. Academician V.I. Goldansky called this transition, thanks to which life on Earth originated, a chiral catastrophe.
How did the conditions arise for the phase catastrophe that caused the chiral transition?
The most important thing was that organic compounds melted at 800-1000 0C in the earth's crust, and the upper ones cooled to the temperature of space, that is, absolute zero. The temperature difference reached 1000 °C. Under such conditions, organic molecules melted under the influence of high temperature and were even completely destroyed, and the top remained cold as the organic molecules were frozen. Gases and water vapor that leaked from the earth's crust changed the chemical composition of organic compounds. The gases carried heat with them, causing the melting line of the organic layer to move up and down, creating a gradient.
At very low atmospheric pressures, water was on the earth's surface only in the form of steam and ice. When the pressure reached the so-called triple point of water (0.006 atmospheres), water was able to exist in the form of a liquid for the first time.
Of course, only experimentally can one prove what exactly caused the chiral transition: terrestrial or cosmic reasons. But one way or another, at some point, chirally ordered molecules (namely, levorotatory amino acids and dextrorotatory sugars) turned out to be more stable and an unstoppable increase in their number began - a chiral transition.
The chronicle of the planet also tells that at that time there were no mountains or depressions on Earth. The semi-molten granitic crust presented a surface as smooth as the level of the modern ocean. However, within this plain there were still depressions due to the uneven distribution of masses within the Earth. These reductions played an extremely important role.
The fact is that flat-bottomed depressions hundreds and even thousands of kilometers across and no more than a hundred meters deep probably became the cradle of life. After all, water that collected on the surface of the planet flowed into them. The water diluted the chiral organic compounds in the ash layer. The chemical composition of the compound gradually changed, and the temperature stabilized. The transition from lifeless to living, which began in anhydrous conditions, continued in an aquatic environment.
Is this the plot of the origin of life? Most likely yes. In the geological section of Isua (Western Greenland), which is 3.8 billion years old, gasoline- and oil-like compounds were found with the C12/C13 isotope ratio characteristic of carbon of photosynthetic origin.
If the biological nature of carbon compounds from the Isua section is confirmed, then it turns out that the entire period of the origin of life on Earth - from the emergence of chiral organic matter to the appearance of a cell capable of photosynthesis and reproduction - was completed in only one hundred million years. And water molecules and DNA played a huge role in this process.
The most amazing thing about the structure of water is that water molecules at low negative temperatures and high pressures inside nanotubes can crystallize into a double helix shape, reminiscent of DNA. This was proven by computer experiments of American scientists led by Xiao Cheng Zeng at the University of Nebraska (USA).

DNA is a double strand twisted into a spiral. Each thread consists of “bricks” - nucleotides connected in series. Each nucleotide of DNA contains one of four nitrogenous bases - guanine (G), adenine (A) (purines), thymine (T) and cytosine (C) (pyrimidines), associated with deoxyribose, to the latter, in turn, a phosphate group is attached . Neighboring nucleotides are connected to each other in a chain by a phosphodiester bond formed by 3"-hydroxyl (3"-OH) and 5"-phosphate groups (5"-PO3). This property determines the presence of polarity in DNA, i.e. opposite directions, namely 5" and 3" ends: the 5" end of one thread corresponds to the 3" end of the second thread. The sequence of nucleotides allows you to “encode” information about various types of RNA, the most important of which are messenger or template (mRNA), ribosomal (rRNA) and transport (tRNA). All these types of RNA are synthesized on a DNA template by copying a DNA sequence into an RNA sequence synthesized during transcription and take part in the most important process of life - the transfer and copying of information (translation).

The primary structure of DNA is the linear sequence of DNA nucleotides in a chain. The sequence of nucleotides in a DNA chain is written in the form of a letter DNA formula: for example - AGTCATGCCAG, the entry is made from the 5" to the 3" end of the DNA chain.
The secondary structure of DNA is formed due to the interactions of nucleotides (mostly nitrogenous bases) with each other, hydrogen bonds. A classic example of DNA secondary structure is the DNA double helix. DNA double helix is the most common form of DNA in nature, consisting of two polynucleotide chains of DNA. The construction of each new DNA chain is carried out according to the principle of complementarity, i.e. Each nitrogenous base of one DNA chain corresponds to a strictly defined base of another chain: in a complementary pair, opposite A is T, and opposite G is C, etc.
In order for water to form a spiral, like this, in a simulated experiment it was “placed” in nanotubes under high pressure, varying in different experiments from 10 to 40,000 atmospheres. After this, the temperature was set, which had a value of -23°C. The margin compared to the freezing point of water was made due to the fact that with increasing pressure the melting point of water ice decreases. The diameter of the nanotubes ranged from 1.35 to 1.90 nm.
 Rice. General view of the structure of water (image by New Scientist)
Rice. General view of the structure of water (image by New Scientist)
Water molecules are connected to each other through hydrogen bonds, the distance between oxygen and hydrogen atoms is 96 pm, and between two hydrogens - 150 pm. In the solid state, the oxygen atom participates in the formation of two hydrogen bonds with neighboring water molecules. In this case, individual H 2 O molecules come into contact with each other with opposite poles. Thus, layers are formed in which each molecule is associated with three molecules of its layer and one from the neighboring one. As a result, the crystal structure of ice consists of hexagonal “tubes” interconnected like a honeycomb.
 Rice. Inner wall of a water structure (New Scientist image)
Rice. Inner wall of a water structure (New Scientist image)
Scientists expected to see that the water in all cases forms a thin tubular structure. However, the model showed that at a tube diameter of 1.35 nm and a pressure of 40,000 atmospheres, the hydrogen bonds were bent, leading to the formation of a double-walled helix. The inner wall of this structure is a quadruple helix, and the outer wall consists of four double helices, similar to the structure of the DNA molecule.
The latter fact leaves an imprint not only on the evolution of our ideas about water, but also on the evolution of early life and the DNA molecule itself. If we assume that in the era of the origin of life, cryolite clay rocks had the shape of nanotubes, the question arises: could the water sorbed in them serve as a structural basis (matrix) for DNA synthesis and information reading? Perhaps this is why the helical structure of DNA repeats the helical structure of water in nanotubes. As New Scientist magazine reports, now our foreign colleagues will have to confirm the existence of such water macromolecules under real experimental conditions using infrared spectroscopy and neutron scattering spectroscopy.
Ph.D. O.V. Mosin
To understand why snowflakes look so beautiful, we need to consider the life history of one snow crystal.
Ice snowflakes in the cloud form at -15 degrees due to the transition of water vapor to a solid state. The basis for the formation of snowflakes are small dust particles or microscopic pieces of ice, which serve as a nucleus for the condensation of water molecules on them. The crystallization nucleus is where the formation of snowflakes begins.

More and more water molecules attach to the growing snowflake in certain places, giving it a distinct hexagonal shape. The key to the structure of solid water lies in the structure of its molecule, which can be simply imagined as a tetrahedron - a pyramid with a triangular base in which angles of only 60° and 120° are possible. In the center there is oxygen, in two vertices there is hydrogen, or more precisely, a proton, the electrons of which are involved in the formation of a covalent bond with oxygen. The two remaining vertices are occupied by pairs of oxygen valence electrons, which do not participate in the formation of intramolecular bonds, which is why they are called lone.

A snowflake is a single crystal of ice, a variation on the theme of a hexagonal crystal, but one that grew quickly under non-equilibrium conditions. Under some conditions, ice hexagons grow intensively along their axis, and then elongated snowflakes are formed - columnar snowflakes, needle snowflakes. Under other conditions, hexagons grow predominantly in directions perpendicular to their axis, and then snowflakes are formed in the form of hexagonal plates or hexagonal stars.

A drop of water can freeze to a falling snowflake, resulting in the formation of snowflakes of irregular shape. The common belief that snowflakes necessarily have the shape of hexagonal stars is erroneous. The shapes of snowflakes turn out to be very diverse.

Astronomer Johannes Kepler wrote a whole treatise “On Hexagonal Snowflakes” in 1611. In 1665, Robert Hooke used a microscope to see and publish many drawings of snowflakes of various shapes. The first successful photograph of a snowflake under a microscope was taken in 1885 by American farmer Wilson Bentley. The most famous followers of Bentley's cause are Ukihiro Nakaya and the American physicist Kenneth Libbrecht. Nakaya was the first to suggest that the size and shape of snowflakes depend on air temperature and moisture content, and brilliantly confirmed this hypothesis experimentally by growing ice crystals of different shapes in the laboratory. And Libbrecht, at Caltech, is still busy growing snowflakes all day long. The scientist, together with photographer Patricia Rasmussen, plan to publish a book that will include the most photogenic snowflakes, some of which can already be seen on his website SnowCrystals.com.

There is another mystery inherent in the structure of a snowflake. In it, order and chaos coexist together. Depending on the production conditions, the solid must be either in a crystalline (when the atoms are ordered) or in an amorphous (when the atoms form a random network) state. Snowflakes have a hexagonal lattice, in which oxygen atoms are arranged in an orderly manner, forming regular hexagons, and hydrogen atoms are arranged randomly. However, the connection between the structure of the crystal lattice and the shape of a snowflake, which is ten million times larger than a water molecule, is not obvious: if water molecules were attached to the crystal in a random order, the shape of the snowflake would be irregular. It's all about the orientation of the molecules in the lattice and the arrangement of free hydrogen bonds, which contributes to the formation of smooth edges.

Water vapor molecules are more likely to fill voids rather than adhere to smooth edges because the voids contain more free hydrogen bonds. As a result, snowflakes take the shape of regular hexagonal prisms with smooth edges. Such prisms fall from the sky, with relatively low air humidity in a wide variety of temperature conditions.

Sooner or later, irregularities appear on the edges. Each tubercle attracts additional molecules and begins to grow. A snowflake travels through the air for a long time, and the chances of meeting new water molecules near the protruding tubercle are slightly higher than at the faces. This is how rays grow on a snowflake very quickly. One thick ray grows from each face, since molecules do not tolerate emptiness. Branches grow from the tubercles formed on this ray. During the journey of a tiny snowflake, all its faces are in the same conditions, which serves as a prerequisite for the growth of identical rays on all six faces. Under ideal laboratory conditions, all six directions of a snowflake grow symmetrically and with similar configurations. In the atmosphere, most snowflakes are irregular crystals; only some of the six branches can be symmetrical.

Nowadays, the study of snowflakes has become a science. Back in 1555, the Swiss explorer Mangus made sketches of the shapes of snowflakes. In 1955, Russian scientist A. Zamorsky divided snowflakes into 9 classes and 48 species. These are plates, needles, stars, hedgehogs, columns, fluffs, cufflinks, prisms, group ones. The International Commission on Snow and Ice adopted a fairly simple classification of ice crystals in 1951: platelets, star-shaped crystals, columns or columns, needles, spatial dendrites, tipped columns and irregular shapes. And three more types of icy precipitation: fine snow pellets, ice pellets and hail.

In 1932, nuclear physicist Ukihiro Nakaya, a professor at Hokkaido University, began growing artificial snow crystals, which made it possible to compile the first classification of snowflakes and identify the dependence of the size and shape of these formations on temperature and air humidity. In the city of Kaga, located on the western coast of the island of Honshu, there is a Museum of Snow and Ice founded by Ukihiro Nakaya, which now bears his name, symbolically built in the form of three hexagons. The museum houses a machine for making snowflakes. Nakaya identified 41 individual morphological types among snowflakes, and meteorologists S. Magano and Xu Li in 1966 described 80 types of crystals.

Under certain conditions, in the absence of wind, falling snowflakes can adhere to each other, forming huge snow flakes. In the spring of 1944, flakes measuring up to 10 centimeters in diameter, similar to whirling saucers, fell in Moscow. And in Siberia, snow flakes with a diameter of up to 30 centimeters were observed. The largest snowflake was recorded in 1887 in Montana, America. Its diameter was 38 cm, and its thickness was 20 cm. This phenomenon requires complete calmness, because the longer the snowflakes travel, the more they collide and adhere to each other. Therefore, at low temperatures and strong winds, snowflakes collide in the air, crumble and fall to the ground in the form of fragments - “diamond dust”. The likelihood of seeing large snowflakes increases significantly near bodies of water: evaporation from lakes and reservoirs is an excellent building material.

The ice that forms a snowflake is transparent, but when there are a lot of them, sunlight, reflected and scattered on numerous faces, gives us the impression of a white opaque mass - we call it snow. The snowflake is white because water absorbs the red and infrared parts of the light spectrum very well. Frozen water largely retains the properties of liquid water. Sunlight, passing through a layer of snow or ice, loses red and yellow rays, which are scattered and absorbed in it, and the light that passes through is bluish-green, blue or bright blue - depending on how thick the layer was in the path of the light .
DATA
Snowflakes form a snow cover that reflects up to 90% of sunlight into space.
In one cubic meter of snow there are 350 million snowflakes, and throughout the entire Earth - 10 to the 24th power.
The weight of the snowflake itself is only about a milligram, rarely 2…3. Nevertheless, by the end of winter, the mass of snow cover in the northern hemisphere of the planet reaches 13,500 billion tons.

By the way, the snow itself is not only white. In arctic and mountainous regions, pink or even red snow is common. This is due to algae living between the crystals. But there are cases when snow fell from the sky already colored. So, on Christmas Day 1969, black snow fell in Sweden. Most likely, this is soot and industrial pollution absorbed from the atmosphere. In 1955, phosphorescent green snow fell near Dana, California, killing several people and causing severe harm to those who tried it on their tongues. There were different versions of this phenomenon, even atomic tests in Nevada. However, they were all rejected and the origin of green snow remained a mystery.

Fresh snow on a frosty day is always accompanied by a cheerful crunch underfoot. This is nothing more than the sound of crystals breaking. Snowflakes also clear the air of dust and fumes, so you can breathe easily during snowfall.

Snowflakes are one of the most beautiful, complex and absolutely unique creations of nature. How are they formed, what are they made of?
Snow is solid precipitation in the form of crystals (snowflakes). There is an exceptionally wide variety of snowflake shapes. The simplest of them are: needles, columns and plates. In addition, there are numerous complicated forms of snowflakes: needle-shaped stars; plate stars; hedgehogs consisting of several columns; columns with plates and stars at the ends. Some column shapes have internal cavities or form the appearance of glasses; 12-pointed stars are also found. The sizes of individual snowflakes can be very different. Needle stars usually have the largest linear dimensions (their radius reaches 4-5 mm). Snowflakes often connect with each other and fall out in the form of flakes. The sizes of flakes can reach very large sizes; flakes with a radius of up to 15-20 cm have been observed. The shape of snowflakes reflects the internal order of water molecules when they are in a solid state - in the form of ice or snow. Snowflakes grow in the same way as crystals of any substance that passes from a liquid to a solid state grow: by connecting with each other, water molecules tend to maximize the forces of mutual attraction and minimize the forces of repulsion, since the energy of the system decreases during crystallization. In just a few minutes, falling on a warm surface, a snowflake will lose its decorative structure, its unique image, which will never be repeated again.




What is snow made of? Both snowflakes and snow crystals are formed from ice. A snow crystal, as its name implies, is a single ice crystal. Snowflake is a more general term; it can refer to an individual snow crystal, multiple snow crystals that stick together, or large clusters of snow crystals that form snow that falls from clouds. Ice crystal structure. Water molecules in an ice crystal form a hexagonal lattice (see figure). Red balls are oxygen atoms. Gray sticks are hydrogen atoms. Two hydrogens for one oxygen - H2O. The sixfold symmetry of snowflakes originates from the crystal lattice of ice. Snowflakes grow from water vapor. Snowflakes are not frozen raindrops. Sometimes raindrops freeze as they fall, but this is called "hail." Hailstones do not have any of the elaborate and symmetrical patterns found in snow crystals. Snow crystals form when water vapor condenses directly into ice, which happens in clouds. Snowflakes are caused by crystal growth. The most basic form of crystalline snow is the hexagonal prism shown above. This structure occurs because certain surfaces of the crystal, the facet surfaces, accumulate material very slowly. This is due to the fact that the surface where the corners are formed is more energetically nonequilibrium than the one that forms the plane, since at the corners there is a greater likelihood of molecules forming bonds with each other. This can be easily demonstrated in a quadrangular crystal, the simplest form. It's the same story with hexagonal prisms. In the photo you can see hexagonal snowflakes collected at the South Pole by Walter Tape. These snowflakes grew quite large because they were frozen for a very long time, which allowed the rule of ice crystal formation to fully manifest itself. A hexagonal prism includes two hexagonal "base" surfaces and six rectangular "prismatic" surfaces, as shown in the figure. Note that a hexagonal prism can be plate-shaped or columnar, depending on the growth rate of the surfaces. When snow crystals are very small, they exist mostly in the form of simple hexagonal prisms. But as they grow, "branches" sprout from the corners of the prisms, creating more complex shapes.


The origin of complex forms of snowflakes. The answer to this question lies in how water molecules move through the air to condense on a growing snow crystal. The molecules diffuse through the air to reach the crystal, and this diffusion slows their growth. More distant water molecules must travel longer through the air to reach the growing crystal. So, consider a flat ice surface that grows in the air. If a small collision occurs and remains on the surface, then the trace from it extends a little further than the rest of the crystal. This means that other water molecules can reach this location faster than the rest of the crystal because they have to travel farther to get there.

As the number of water molecules reaching the collision site increases, the collision site grows faster. After a short time, collisions occur more frequently, and growth occurs even faster. Then what is called branching instability occurs - new small collisions originate on large branches, and become the site of formation of side branches. This is how complexity is born. This instability is the main reason for the creation of complex snow crystal shapes.




When branching instability is applied to a snow crystal over and over again, the result is what is called an ice dendrite. The word "dendrite" means "tree-like", and star-shaped, tree-like snow crystals are common. The rate of diffusion of water molecules can be changed in the laboratory. If snow crystals are grown in air below atmospheric pressure, they are less branched. This is because diffusion does not limit growth at low pressure, therefore branching instability is not as intense. At higher pressures, more branched snow crystals form. The growth of snow crystals depends on the balance between edges and branching. Edges tend to create simple flat surfaces, while branches tend to create more complex structures. The interaction between edges and branching is subtle and highly dependent on parameters such as temperature and humidity. This means that snow crystals can grow in many different ways, resulting in the great variety seen in snowflake shapes.



The famous astronomer Johannes Kepler was the first to study snowflakes. In 1611, he published a treatise “On Hexagonal Snowflakes,” in which he mainly discussed the geometric aspects of their structure. We had to wait more than two centuries for the next breakthrough. For his 15th birthday, his mother gave her son, a young farmer from Vermont, Wilson Alvin Bentley, a microscope. And he decided to look at the snowflakes through it. On January 15, 1885, he took the first photograph of a snowflake by attaching a camera to a microscope and photographing it against a background of black paper. By the end of his life, he had captured images of 5,381 snowflakes. In 1920, he received a position in the National Weather Service and a $25 grant for his research, and snow began to fall not only on farms, but also in the laboratories of kineticists and crystallographers. But it was Bentley who was the first to say that he had never seen two identical snowflakes. There is a common belief that there are no two identical snowflakes in nature. It would seem how it could be. Millions are falling from the sky. But, on the other hand, if you estimate very roughly, then there are approximately 1020 water molecules in a snowflake, and the human eye is able to determine about 100 visual parameters of a snowflake. So such a mosaic can come together in a finite but insanely huge number of ways. And if you remember that oxygen and hydrogen atoms have different isotopes, and there are still impurities in water... in general, it’s worth accepting that no two snowflakes are alike in nature. But the crystals have a symmetrical shape. Macroscopic factors (temperature, pressure, concentrations of various substances) in such a small space as the current position of the crystal nucleus at a time do not differ much, and growth in all directions is the same. Until a break occurs or, conversely, sticking occurs.
It is in a state of aggregation, which tends to have a gaseous or liquid form at room temperature. The properties of ice began to be studied hundreds of years ago. About two hundred years ago, scientists discovered that water is not a simple compound, but a complex chemical element consisting of oxygen and hydrogen. After discovery, the formula of water became H2O.
Ice structure
H 2 O consists of two hydrogen atoms and one oxygen atom. In a quiet state, hydrogen is located on the tops of the oxygen atom. Oxygen and hydrogen ions should occupy the vertices of an isosceles triangle: oxygen is located at the vertex of a right angle. This structure of water is called a dipole.
Ice consists of 11.2% hydrogen, and the rest is oxygen. The properties of ice depend on its chemical structure. Sometimes it contains gaseous or mechanical formations - impurities.
Ice occurs in nature in the form of a few crystalline species that stably retain their structure at temperatures from zero and below, but at zero and above it begins to melt.
Crystal structure
The properties of ice, snow and steam are completely different and depend on In the solid state, H 2 O is surrounded by four molecules located at the corners of the tetrahedron. Since the coordination number is low, the ice may have an openwork structure. This is reflected in the properties of ice and its density.

Ice shapes
Ice is one of the most common substances in nature. On Earth there are the following varieties:
- river;
- lake;
- nautical;
- firn;
- glacier;
- ground.
There is ice that is directly formed by sublimation, i.e. from the vapor state. This appearance takes on a skeletal shape (we call them snowflakes) and aggregates of dendritic and skeletal growth (frost, hoarfrost).
One of the most common forms are stalactites, i.e. icicles. They grow all over the world: on the surface of the Earth, in caves. This type of ice is formed by the flow of water droplets when the temperature difference is about zero degrees in the autumn-spring period.
Formations in the form of ice strips that appear along the edges of reservoirs, at the boundary of water and air, as well as along the edge of puddles, are called ice banks.
Ice can form in porous soils in the form of fibrous veins.
Properties of ice
A substance can be in different states. Based on this, the question arises: what property of ice is manifested in this or that state?
Scientists distinguish physical and mechanical properties. Each of them has its own characteristics.

Physical properties
The physical properties of ice include:
- Density. In physics, an inhomogeneous medium is represented by the limit of the ratio of the mass of the substance of the medium itself to the volume in which it is contained. The density of water, like other substances, is a function of temperature and pressure. Typically, calculations use a constant density of water equal to 1000 kg/m3. A more accurate density indicator is taken into account only when it is necessary to carry out very accurate calculations due to the importance of the resulting density difference result.
When calculating the density of ice, it is taken into account what kind of water has become ice: as is known, the density of salt water is higher than distilled water. - Water temperature. Usually occurs at a temperature of zero degrees. Freezing processes occur intermittently with the release of heat. The reverse process (melting) occurs when the same amount of heat is absorbed that was released, but without jumps, but gradually.
In nature, there are conditions under which water is supercooled, but it does not freeze. Some rivers retain liquid water even at a temperature of -2 degrees. - the amount of heat that is absorbed when a body is heated by each degree. There is a specific heat capacity, which is characterized by the amount of heat required to heat a kilogram of distilled water by one degree.
- Compressibility. Another physical property of snow and ice is compressibility, which affects the decrease in volume under the influence of increased external pressure. The reciprocal quantity is called elasticity.
- Ice strength.
- Ice color. This property depends on the absorption of light and the scattering of rays, as well as the amount of impurities in the frozen water. River and lake ice without foreign impurities is visible in soft blue light. Sea ice can be completely different: blue, green, blue, white, brown, or have a steely tint. Sometimes you can see black ice. It acquires this color due to a large number of minerals and various organic impurities.

Mechanical properties of ice
The mechanical properties of ice and water are determined by their resistance to the influence of the external environment relative to a unit area. Mechanical properties depend on structure, salinity, temperature and porosity.
Ice is an elastic, viscous, plastic formation, but there are conditions under which it becomes hard and very brittle.
Sea ice and freshwater ice are different: the former is much more flexible and less durable.

When passing ships, the mechanical properties of ice must be taken into account. This is also important when using ice roads, crossings and more.
Water, snow and ice have similar properties that determine the characteristics of the substance. But at the same time, these readings are influenced by many other factors: ambient temperature, impurities in the solid, as well as the initial composition of the liquid. Ice is one of the most interesting substances on Earth.
In this article we will talk about the structure of water molecules, their connections and properties.
Jumping ahead a little, I’ll write:
The task performed by the Mayer Cell is the “easy” decomposition of water molecules under the influence of an electric current accompanied by electromagnetic radiation.
To solve it, let’s figure out what water is? What is the structure of water molecules? What is known about water molecules and their bonds? In the article, I used various publications that are available in sufficient quantities on the Internet, but they are reproduced in large quantities, so it is not clear to me who their author is and it is stupid for me to cite a source. Moreover, these publications are “confused” to the point of disgrace, which makes it difficult to understand and significantly increases the study time. By analyzing the articles, I extracted something that can guide you in understanding what we will be dealing with in the process of extracting cheap energy, or more precisely in the process of breaking water molecules into components - hydrogen and oxygen.
So, let's look at the most important concepts about the structure of water molecules!
Water is a substance whose main structural unit is the H 2 O molecule, consisting of one oxygen atom and two hydrogen atoms.
The water molecule has the structure of an isosceles triangle: at the top of this triangle there is an oxygen atom, and at its base there are two hydrogen atoms. The apex angle is 104°27, and the side length is 0.096 nm. These parameters refer to the hypothetical equilibrium state of a water molecule without its vibrations and rotations. The geometry of the water molecule and its electron orbits are shown in the figure.
A water molecule is a dipole containing positive and negative charges at its poles. If a “free” water molecule, not connected to other molecules, is placed in an electric field, then it will “turn” with its negative poles towards the positive plate of the electric field, and with its positive poles towards the negative plate. It is this process that is depicted in Figure 1, position 3B, explaining the operation of the Mayer Cell in the article “Water instead of gasoline”.
 If you connect the epicenters of positive and negative charges with straight lines, you get a three-dimensional geometric figure - a regular tetrahedron. This is the structure of the water molecule itself.
If you connect the epicenters of positive and negative charges with straight lines, you get a three-dimensional geometric figure - a regular tetrahedron. This is the structure of the water molecule itself.
Due to the presence of hydrogen bonds, each water molecule forms a hydrogen bond with 4 neighboring molecules, forming an openwork mesh frame in the ice molecule. It is this ordered state of water molecules that can be called “structure.” Each molecule can simultaneously form four hydrogen bonds with other molecules at strictly defined angles equal to 109°28′, directed towards the vertices of the tetrahedron, which do not allow the creation of a dense structure during freezing.
 When ice melts, its tetragonal structure breaks down and a mixture of polymers is formed, consisting of tri-, tetra-, penta-, and hexamers of water and free water molecules.
When ice melts, its tetragonal structure breaks down and a mixture of polymers is formed, consisting of tri-, tetra-, penta-, and hexamers of water and free water molecules.
In the liquid state, water is a disordered liquid. These hydrogen bonds are spontaneous, short-lived, quickly break and form again.
When grouped, the tetrahedra of water molecules form a variety of spatial and planar structures.
And of all the variety of structures in nature, the basic one is the hexagonal (six-sided) structure, when six water molecules (tetrahedra) are combined into a ring.
 This type of structure is characteristic of ice, snow and melt water, which, due to the presence of such a structure, is called “Structured water”. Much has been written about the beneficial properties of structured water, but this is not the topic of our article. It would be logical that structured water—forming hexagonal structures—is the worst option for the structure of water, which can be used for decomposition into hydrogen and oxygen. Let me explain why: Water molecules, grouped six into a hexamer, have an electrically neutral composition - hexamers do not have positive and negative poles. If you place a hexamer of structured water in an electric field, it will not react to it in any way. Therefore, it can be logically concluded that it is necessary for the water to have as few organized structures as possible. In fact, it’s the other way around: a hexamer is not a complete structure; there is an even more interesting concept - a cluster.
This type of structure is characteristic of ice, snow and melt water, which, due to the presence of such a structure, is called “Structured water”. Much has been written about the beneficial properties of structured water, but this is not the topic of our article. It would be logical that structured water—forming hexagonal structures—is the worst option for the structure of water, which can be used for decomposition into hydrogen and oxygen. Let me explain why: Water molecules, grouped six into a hexamer, have an electrically neutral composition - hexamers do not have positive and negative poles. If you place a hexamer of structured water in an electric field, it will not react to it in any way. Therefore, it can be logically concluded that it is necessary for the water to have as few organized structures as possible. In fact, it’s the other way around: a hexamer is not a complete structure; there is an even more interesting concept - a cluster.
The structures of united water molecules are called clusters, and individual water molecules are called quanta. A cluster is a volumetric combination of water molecules, including hexamers, which has both positive and negative poles.
In distilled water, clusters are practically electrically neutral, because as a result of evaporation, the clusters were destroyed, and as a result of condensation, strong bonds between water molecules did not appear. However, their electrical conductivity can be changed. If distilled water is stirred with a magnetic stirrer, the connections between the elements of the clusters will be partially restored and the electrical conductivity of the water will change. In other words, distilled water is water that has a minimum number of bonds between molecules . In it, the dipoles of the molecules are in a misoriented state, so the dielectric constant of distilled water is very high, and it is a poor conductor of electric current. At the same time, to increase the controllability of water clusters, acids or alkalis are added to it, which, by participating in molecular bonds, do not allow water molecules to form hexagonal structures, thereby forming electrolytes. Distilled water is the opposite of structured water, in which there are a huge number of connections between water molecules in clusters.
On my website there are, and will continue to appear, articles that, at first glance, are “separate” and have no relation to other articles. In fact, most of the articles on the site are interconnected into one whole. In this case, when describing the properties of distilled water, I use the Dipole theory of electric current, this is an alternative concept of electric current, which is confirmed by both science and practice better than the classical concept.
When exposed to the energy of an electric current source, all dipoles of water atoms (as a conductor) rotate, oriented with their like poles in one direction. If water molecules created a cluster (mutually oriented) structure before the appearance of an external electric field, then for orientation in an external electric field a minimum amount of energy from an electric current source will be required. If the structure was not organized (like distilled water), then a large amount of energy will be required.
Please note, “there is a popular opinion” that distilled water and melt water should have the same electrical conductive properties, because both one and the other have no chemical impurities (usually salts), their chemical composition is the same, and the structure of water molecules is the same in melt water, it is the same in distilled water.
In fact, everything looks the other way around; the absence of impurities does not at all indicate the electrical conductivity properties of water. Without understanding this, some people “kill” batteries even at the stage of filling them with electrolyte, replacing distilled water with melt water, or simply purified through a carbon filter. As a rule, a refilled battery that was purchased on the automotive market lasts less than one that you bought dry-charged and diluted with sulfuric acid with distilled water and refilled it yourself. This is only because a “ready” electrolyte, or a refilled battery, is a means of earning money in our time, and in order to determine what kind of water was used, an expensive examination must be carried out, no one bothers with this. The dealer doesn’t care how long the battery in your car will last, and you don’t really want to mess around with acid either. But, I assure you, the battery you sweat over will be much more vigorous at subzero temperatures than one filled with ready-made bottled electrolyte.
Let's continue!
In water, clusters periodically collapse and form again. The jump time is 10 -12 seconds.
Since the structure of the water molecule is asymmetrical, the centers of gravity of its positive and negative charges do not coincide. Molecules have two poles - positive and negative, creating, like a magnet, molecular force fields. Such molecules are called polar or dipoles, and the quantitative characteristic of polarity is determined by the electric moment of the dipole, expressed as the product of the distance l between the electrical centers of gravity of the positive and negative charges of a molecule per charge e in absolute electrostatic units: p = l e
For water, the dipole moment is very high: p = 6.13·10 -29 C m.
Water clusters at the phase boundaries (liquid-air) are arranged in a certain order, with all clusters oscillating at the same frequency, acquiring one common frequency. With such movement of clusters, taking into account that the water molecules included in the cluster are polar, that is, they have a large dipole moment, we should expect the appearance of electromagnetic radiation. This radiation differs from the radiation of free dipoles, since the dipoles are coupled and oscillate together in a cluster structure.
The frequency of oscillations of water clusters and, accordingly, the frequency of electromagnetic oscillations can be determined by the following formula:
 Where a
— surface tension of water at a given temperature; M
Where a
— surface tension of water at a given temperature; M
— mass of the cluster.
Where V — cluster volume.
The volume of the cluster is determined taking into account the dimensions of the fractal closed structure of the cluster or by analogy with the dimensions of the protein domain.
At room temperature 18°C, the cluster oscillation frequency f
equal to 6.79 10 9 Hz, that is, the wavelength in free space should be λ
= 14.18 mm.
 But what will happen when water is exposed to external electromagnetic radiation? Since water is a self-organized structure and contains both elements ordered into clusters and free molecules, the following will happen when exposed to external electromagnetic radiation. When water molecules come closer (the distance changes from R 0 to R 1 ), the interaction energy changes by a greater amount than when they move away from each other (the distance changes from R 0 to R 2 ).
But what will happen when water is exposed to external electromagnetic radiation? Since water is a self-organized structure and contains both elements ordered into clusters and free molecules, the following will happen when exposed to external electromagnetic radiation. When water molecules come closer (the distance changes from R 0 to R 1 ), the interaction energy changes by a greater amount than when they move away from each other (the distance changes from R 0 to R 2 ).
But, since water molecules have a large dipole moment, in the case of an external electromagnetic field, they will perform oscillatory movements (for example, from R 1 to R 2 ). In this case, due to the above dependence, the applied electromagnetic field will contribute more to the attraction of molecules and thereby the organization of the system as a whole, i.e. formation of a hexagonal structure.
If there are impurities in the aqueous environment, they are covered with a hydration shell in such a way that the total energy of the system tends to take a minimum value. And if the total dipole moment of the hexagonal structure is zero, then in the presence of impurities, the hexagonal structure near them is disrupted in such a way that the system takes on a minimum value; in some cases, hexagons are transformed into pentagons, and the hydration shell has a shape close to a ball. Impurities (for example, Na + ions) can stabilize the structure, making it more resistant to destruction.
A self-organized system of water, when exposed to electromagnetic radiation, will not move as a single whole, but each element of the hexagonal structure, and in the case of locally impurities of another type, will shift, i.e. the geometry of the structure will be distorted, i.e. tensions arise. This property of water is very similar to polymers. But polymer structures have long relaxation times, which are not 10 -11 –10 -12 s, but minutes or more. That's why the energy of electromagnetic radiation quanta, turning into the internal energy of an organized water structure as a result of its distortions, will be accumulated by it until it reaches the hydrogen bond energy, which is 500–1000 times greater than the energy of the electromagnetic field. When this value is reached, the hydrogen bond breaks and the structure collapses.
This can be compared to a snow avalanche, when there is a gradual, slow accumulation of mass, and then a rapid collapse. In the case of water, not only the weak bonds between clusters are broken, but also stronger bonds in the structure of water molecules. As a result of this rupture, H +, OH –, and hydrated electron e – can be formed. The blue color of pure water is due to the presence of these electrons, and not just the scattering of natural light.
Conclusion
Thus, when exposed to electromagnetic radiation with water, energy accumulates in the cluster structure to a certain critical value, then bonds between clusters and others are broken, and an avalanche-like release of energy occurs, which can then be transformed into other types.
In the next article, “The breaking of water molecules into hydrogen and oxygen. Ohm's Law and Mayer's Cell”, we will determine the conditions for the rupture of water molecules and figure out how Ohm’s Law interferes with “our desires.”