Solutions play a key role in nature, science and technology. Water is the basis of life, it always contains solutes. Fresh water of rivers and lakes contains few dissolved substances, while sea water contains about 3.5% dissolved salts.
The primary ocean (at the time of the origin of life on Earth), according to assumptions, contained only 1% of dissolved salts.
“It was in this environment that living organisms first developed, from this solution they scooped ions and molecules that are necessary for their further growth and development ... Over time, living organisms developed and transformed, so they were able to leave the aquatic environment and move to land and then rise to air. They obtained these abilities by storing an aqueous solution in their organisms in the form of liquids, which contain a vital supply of ions and molecules "- these are the words that the famous American chemist and Nobel Prize winner Linus Pauling describes the role of solutions in nature. Inside each of us, in every cell of our body, there are memories of the primary ocean, the place in which life originated, - an aqueous solution that provides life itself.
In any living organism, an unusual solution constantly flows through the vessels - arteries, veins and capillaries, which forms the basis of blood, the mass fraction of salts in it is the same as in the primary ocean - 0.9%. Complex physicochemical processes occurring in the human and animal body also interact in solutions. The process of assimilation of food is associated with the transfer of highly nutritious substances into solution. Natural aqueous solutions are directly related to the processes of soil formation, supply of plants nutrients... Such technological processes in the chemical and many other industries, for example, the production of fertilizers, metals, acids, paper, occur in solutions. Modern science is studying the properties of solutions. Let's find out what is a solution?
 Solutions differ from other mixtures in that the particles of the constituent parts are evenly distributed in them, and the composition will be the same in any microvolume of such a mixture.
Solutions differ from other mixtures in that the particles of the constituent parts are evenly distributed in them, and the composition will be the same in any microvolume of such a mixture.
That is why solutions were understood as homogeneous mixtures that consist of two or more homogeneous parts. This idea was based on the physical theory of solutions.
The adherents of the physical theory of solutions, which were occupied by Van't Hoff, Arrhenius and Ostwald, believed that the process of dissolution is the result of diffusion.
DI Mendeleev and supporters of the chemical theory believed that dissolution is the result of the chemical interaction of a solute with water molecules. Thus, it will be more accurate to define a solution as a homogeneous system, which consists of particles of a solute, a solvent, and the products of their interaction.
Due to the chemical interaction of a solute with water, compounds are formed - hydrates. Chemical interactions are usually accompanied by thermal phenomena. For example, the dissolution of sulfuric acid in water takes place with the release of such a colossal amount of heat that the solution can boil, which is why the acid is poured into the water, and not vice versa. Dissolution of substances such as sodium chloride, ammonium nitrate is accompanied by heat absorption.
MV Lomonosov proved that solutions turn into ice at a lower temperature than a solvent.
www.site, with full or partial copying of the material, a link to the source is required.
Topic: Water and solutions.
Plan:
- Water in nature. Water properties.
- Solutions.
- Ways of expressing the composition of the solution.
- Hydrates and crystalline hydrates.
- Solubility.
- Supersaturated solutions.
- Osmosis.
- Vapor pressure, solutions.
- Freezing and boiling of solutions.
- Buffer solutions.
Water is "a very widespread substance on Earth. Almost threefourth surfaces of the globe are covered with water, forming oceans, seas, rivers and lakes. A lot of water is in a gaseous state as vapor in the atmosphere; in the form of huge masses of snow and ice, it lies all year round on the tops of high mountains and in polar countries. There is also water in the bowels of the earth, soaking the soil and rocks.
Natural water is never completely clean. The purest is rainwater, but it also contains small amounts of various impurities that it captures from the air.
The amount of impurities in fresh waters usually ranges from 0.01 to 0.1% (mass). Sea water contains 3.5% (mass) of dissolved substances, the main mass of which is sodium chloride (table salt).
Water that contains significant amounts of calcium and magnesium salts is called hard water, as opposed to soft water such as rainwater. Hard water gives little foam with soap, and forms scale on the walls of boilers.
To free natural water from particles suspended in it, it is filtered through a layer of porous substance, for example, coal, fired clay, etc. When filtering large amounts of water, filters made of sand and gravel are used. The filters also trap most of the bacteria. In addition, for disinfection drinking water it is chlorinated; complete sterilization of water requires no more than 0.7 g of chlorine per 1 ton of water.
Filtration can remove only insoluble impurities from the water. Dissolved substances are removed from it by distillation (distillation) or ion exchange.
Water is very important in the life of plants, animals and humans. According to modern ideas, the very origin of life is associated with the sea. In any organism, water is an environment in which chemical processes take place that ensure the vital activity of the organism; in addition, she herself takes part in a number of biochemical reactions.
Physical properties of water. Pure water is a colorless transparent liquid. The density of water during the transition from a solid state to a liquid does not decrease, as almost
for all other substances, but increases. When water is heated from 0 to 4 ° C, its density also increases. At 4 ° C, water has a maximum density, and only with further heating does its density decrease.
Of great importance in the life of nature is the fact that water has an abnormally high heat capacity.
Due to the fact that when ice melts, the volume occupied by water decreases, the pressure lowers the melting point of ice. This follows from Le Chatelier's principle. Thus, an increase in pressure at 0 ° C causes the transformation of ice into a liquid, which means that the melting point of ice decreases.
The water molecule has an angular structure; the nuclei included in its composition form an isosceles triangle, at the base of which there are two protons, and at the apex — the nucleus of the oxygen atom. The O – H internuclear distances are close to 0.1 nm, the distance between the nuclei of hydrogen atoms is approximately 0.15 nm. Of the eight electrons that make up the outer electron layer of the oxygen atom in the water molecule, two electron pairs form covalent O – H bonds, and the remaining four electrons are two unshared electron pairs.
The oxygen atom in the water molecule is in the 5p state 3 -hybridization. Therefore, the bond angle НОН (104.3 °) is close to tetrahedral (109.5 °). The electrons forming the O — H bonds are displaced toward the more electronegative oxygen atom. As a result, hydrogen atoms acquire effective positive charges, so that two positive poles are created on these atoms. The centers of negative charges of the lone electron pairs of the oxygen atom located on the hybrid 5p 3 -orbitals are displaced relative to the atomic nucleus and create two negative poles.
The molecular weight of vaporous water is 18 units. But the molecular weight of liquid water, determined by studying its solutions in other solvents, turns out to be higher, higher. This is due to the fact that in liquid water there is an association of individual water molecules into more complex aggregates (clusters). This conclusion is also confirmed by the abnormally high values of the melting and boiling points of water. The association of water molecules is caused by the formation of hydrogen bonds between them.
By its structure, water is a hierarchy of regular volumetric structures, which are based on crystal-like formations, consisting of 57 molecules and interacting with each other due to free hydrogen bonds. This leads to the appearance of second-order structures in the form of hexagons, consisting of 912 water molecules. The properties of clusters depend on the ratio of oxygen and hydrogen to the surface. The configuration of water elements reacts to any external influence and impurities, which explains the extremely labile nature of their interaction. In ordinary water, the aggregate of individual water molecules and random associates is 60% (destructured water), and 40% are clusters (structured water).
In solid water (ice), the oxygen atom of each molecule participates in the formation of two hydrogen bonds with neighboring water molecules. The formation of hydrogen bonds leads to such an arrangement of water molecules in which they touch each other with their opposite poles. The molecules form layers, each of which is associated with three molecules belonging to the same layer, and with one from an adjacent layer. The structure of ice belongs to the least dense structures, there are voids in it, the dimensions of which are somewhat larger than the size of the molecule.
When ice melts, its structure is destroyed. But even in liquid water, hydrogen bonds between molecules are preserved: associates are formed - fragments of ice structures - consisting of a larger or smaller number of water molecules. However, unlike ice, each associate exists for a very short time: the destruction of some and the formation of other aggregates constantly occurs. Single water molecules can be located in the voids of such “ice” aggregates; in this case, the packing of water molecules becomes denser. That is why, when ice melts, the volume occupied by water decreases, and its density increases.
As the water heats up, the debris of the ice structure in it becomes less and less, which leads to a further increase in water density. In the temperature range from 0 to 4 ° C, this effect prevails over thermal expansion, so that the density of water continues to increase. However, when heated above 4 ° C, the effect of increased thermal motion of molecules predominates and the density of water decreases. Therefore, at 4 ° C, water has a maximum density.
When water is heated, part of the heat is spent on breaking hydrogen bonds (the energy of breaking a hydrogen bond in water is about 25 kJ / mol). This explains the high heat capacity of water. Hydrogen bonds between water molecules are completely broken only when water passes into steam.
The state diagram of water (or phase diagram) is a graphical representation of the relationship between the quantities characterizing the state of the system and phase transformations in the system (transition from solid to liquid, from liquid to gaseous, etc.). For one-component systems, phase diagrams are usually used, showing the dependence of phase transformations on temperature and pressure; they are called P-T state diagrams.
At the temperature corresponding to this point, the critical temperature, the quantities characterizing the physical properties of the liquid and vapor become the same, so that the difference between the liquid and vapor states disappears.
The existence of a critical temperature was established in 1860 by D.I.Mendeleev, studying the properties of liquids. He showed that at temperatures above the critical one, a substance cannot be in a liquid state. In 1869 Andrews, studying the properties of gases, came to a similar conclusion.
The critical temperature and pressure for different substances are different. So, for hydrogen = -239.9 ° C, = 1.30 MPa, for chlorine = 144 ° C, = 7.71 MPa, for water = 374.2 ° C, = 22.12 MPa.
Water molecules are highly resistant to heat. However, at temperatures above 1000 ° C, water vapor begins to decompose into hydrogen and oxygen constituting water. The process of decomposition of a substance as a result of its heating is called thermal dissociation. Thermal dissociation of water occurs with the absorption of heat. Therefore, according to the principle of equilibrium of the French scientist Le Chatelier, the higher the temperature, the more the water decomposes. However, even at 2000 ° C, the degree of thermal dissociation of water does not exceed 2%, i.e. the equilibrium between gaseous water and the products of its dissociation - hydrogen and oxygen - still remains shifted towards water. On cooling below 1000 ° C, the equilibrium is almost completely shifted in this direction.
Water is a highly reactive substance. Oxides of many metals and non-metals combine with water to form bases and acids; some salts form crystalline hydrates with water; the most active metals interact with water with the evolution of hydrogen.
Water also has catalytic properties. In the absence of traces of moisture, some of the usual reactions practically do not occur; for example, chlorine does not interact with metals, hydrogen fluoride does not corrode glass, sodium does not oxidize in air.
Water is able to combine with a number of substances that are under normal conditions in a gaseous state, forming the so-called gas hydrates. Examples are compounds of xenon, chlorine and hydrocarbons, which precipitate in the form of crystals at temperatures from 0 to 24 ° C (usually at an elevated pressure of the corresponding gas). Such compounds arise as a result of the filling of intermolecular cavities with gas molecules (“guest”) in the structure of water (“host”); they are called inclusion compounds or clathrates.
In clathrate compounds, only weak intermolecular bonds are formed between the “guest” and “host” molecules; the included molecule cannot leave its place in the crystal cavity, mainly due to spatial difficulties. Therefore, clathrates are unstable compounds that can exist only at relatively low temperatures.
Clathrates are used to separate hydrocarbons and noble gases. Recently, the formation and destruction of gas clathrates (propane and some others) has been successfully used for water demineralization. By injecting an appropriate gas into salt water at elevated pressure, ice-like clathrate crystals are obtained, while the salts remain in solution. The snow-like mass of crystals is separated from the mother liquor and washed. Then, with a slight increase in temperature or a decrease in pressure, the clathrates decompose, forming fresh water and the original gas, which is again used to obtain the clathrate. High efficiency and relatively mild conditions for the implementation of this process make it promising as an industrial method of desalination of sea water.
A solution is a solid or liquid homogeneous system consisting of two or more components (constituents), the relative amounts of which can vary within wide limits.
Every solution consists of solutes and a solvent, i.e. environment in which these substances are evenly distributed in the form of molecules or ions. Usually, the solvent is considered to be the component that exists in its pure state in the same state of aggregation as the resulting solution (for example, in the case of an aqueous salt solution, the solvent is, of course, water). If both components were in the same state of aggregation before dissolution (for example, alcohol and water), then the component that is in a larger amount is considered the solvent.
The homogeneity of the solutions makes them very similar to chemical compounds. The release of heat during dissolution of some substances also indicates a chemical interaction between the solvent and the substance being dissolved. The difference between solutions and chemical compounds is that the composition of the solution can vary within wide limits.
In addition, many properties of its individual components can be found in the properties of a solution, which is not observed in the case of a chemical compound. The inconstancy of the composition of solutions brings them closer to mechanical mixtures, but they differ sharply from the latter in their homogeneity.
Thus, solutions occupy an intermediate position between mechanical mixtures and chemical compounds.
The dissolution of a crystal in a liquid proceeds as follows. When a crystal is introduced into a liquid in which it can dissolve, individual molecules are detached from its surface. The latter, due to diffusion, are evenly distributed throughout the volume of the solvent. The separation of molecules from the surface of a solid is caused, on the one hand, by their own vibrational motion, and, on the other, by attraction from the solvent molecules.
Then a dynamic equilibrium is established, in which as many molecules dissolve per unit time as they are released from the solution.
A solution in equilibrium with races a dissolving substance is called a saturated solution.
Saturated solutions are used relatively rarely. In most cases, unsaturated solutions are used that contain less solute than a saturated solution contains at a given temperature. In this case, solutions with a low solute content are called dilute, with a high one - concentrated.
The composition of the solution (and, in particular, the content of the solute in it) can be expressed different ways- both with the help of dimensionless units (fractions or percentages), and through dimensional quantities - concentration.
Ways of expressing the composition of solutions
|
Name and symbol |
Definition |
Dimension |
Note |
|
|
Mass fraction of solute B, w (B) |
The ratio of the mass of the solute B (mB) to the mass of the solution (mP). |
Dimensionless quantity |
|
in 100 mass.h. the solution contains 20 mass. including NaOH |
|
Molar fraction of solute B, xB |
The ratio of the amount of the second substance (nB) to the total amount of all substances that make up the solution, including the solvent (е ni = nB + n1 + n2 + ... ni) |
Dimensionless quantity |
xHCl = 0.02 or xHCl = 2% - |
|
|
Molarity of substance B in solution, Cm (B) |
The ratio of the amount of solute B (nB) to the mass of solvent (mB) in kg |
mol / kg = Mn |
|
C m (H 2 SO 4) = 0.1 mol / kg C m (H 2 SO 4) = 0.1 Mn in solution for 1 kg of H2 O there is 0.1 mol of H2 SO4. The solution is called decimolar |
|
Molar concentration of substance B, CB |
The ratio of the amount of solute B (nB) to the volume of the solution (VР) |
mol / L = M |
|
C (KCl) = 2 mol / l 1 liter of solution contains 2 mol of KCl |
|
Molar concentration of equivalents of substance B, Ceq (B) |
The ratio of the number of equivalents of the solute B (neq) to the volume of the solution (VР) |
mol / L = n |
|
C eq (Na 2 CO 3) = 0.01 mol / l C eq (Na 2 CO 3) = 0.01 n 1 liter of solution contains 0.01 mol of Na2 CO3 equivalents - centimolar solution |
|
The product of the molar concentration of equivalents of substance B (Ceq (B)) by the volume of solution (VР) is equal to the number of equivalents of this substance (neq (B)). Therefore, the law of equivalents: neq (A) + neeq (B) for solutions has the form: C eq (A) V P (A) = C eq (B) V P (B). This equation is very often used in calculations, especially in analytical chemistry. |
||||
|
Substance B solution titer |
Concentration of a standard solution equal to the mass of substance B (mB) contained in 1 ml of solution |
|
T (NaCl) = 0.0250 g / ml 1 ml of solution contains 0.0250 g of NaCl |
|
Most substances in a crystalline state dissolve in liquids with heat absorption. However, when sodium hydroxide, potassium carbonate, anhydrous copper sulfate and many other substances are dissolved in water, a noticeable increase in temperature occurs. Heat is also released when some liquids and all gases are dissolved in water.
The amount of heat absorbed (or released) during the dissolution of one mole of a substance is called the heat of dissolution of this substance.
The heat of dissolution has a negative value if heat is absorbed during dissolution, and positive value when heat is released. For example, the heat of dissolution of ammonium nitrate is -26.4 kJ / mol, potassium hydroxide +55.6 kJ / mol, etc.
The dissolution process is accompanied by a significant increase in the entropy of the system, since as a result of the uniform distribution of particles of one substance in another, the number of microstates of the system sharply increases. Therefore, despite the endothermicity of dissolution of most crystals, the change in the Gibbs energy of the system during dissolution is negative and the process proceeds spontaneously.
When crystals dissolve, their destruction occurs, which requires energy consumption. Therefore, dissolution would have to be accompanied by heat absorption. If the opposite effect is observed, then this shows that simultaneously with dissolution, some interaction occurs between the solvent and the dissolved substance, in which more energy is released in the form of heat than it is consumed and the destruction of the crystal lattice.
Indeed, it has now been established that when many substances dissolve, their molecules (or ions) with solvent molecules, forming compounds called solvatam and (from Latin solvere - dissolve); this process is called solvation. In the particular case, when the solvent is water, these compounds are called hydrates, and the very process of their formation is called hydration.
Hydrates, as a rule, are unstable compounds, which in many cases decompose already upon evaporation of solutions. But sometimes hydrates are so strong that when a solute is released from a solution, water is part of its crystals. Substances, the crystals of which include water molecules, are called crystalline hydrates, and the water contained in them is called p and -. with t and l l and z and z and about and n about th.
The composition of crystalline hydrates is usually depicted by formulas showing how much crystallization water contains crystalline hydrate. For example, crystalline copper sulfate hydrate (copper sulfate).
The bond strength between the substance and crystallization water in crystalline hydrates is different. Many of them lose crystallization water already at room temperature. Thus, transparent crystals of soda (NaC0 3 - 10H 2 O) easily "erode" - losing crystallization water, become dull and gradually crumble into powder. Quite strong heating is required to dehydrate other crystalline hydrates.
Solubility is the ability of a substance to dissolve in a particular solvent. A measure of the solubility of a substance under given conditions is its content in a saturated solution. Therefore, numerically solubility can be expressed in the same ways as the composition, for example, the percentage of the mass of a solute to the mass of a saturated solution or the amount of a solute contained in 1 liter of a saturated solution. Solubility is often expressed also by the number of mass units of an anhydrous substance, saturating under given conditions 100 mass units of the solvent; sometimes the solubility expressed in this way is called the coefficient of solubility.
The solubility of various substances in water varies widely. If more than 10 g of a substance is dissolved in 100 g of water, then such a substance is usually called highly soluble; if less than 1 g of a substance dissolves, it is poorly soluble and, finally, practically insoluble, if less than 0.01 g of a substance passes into the solution.
The dissolution of most solids is accompanied by the absorption of heat. This is due to the expenditure of a significant amount of energy to destroy the crystal lattice of a solid, which is usually not fully compensated for by the energy released during the formation of hydrates (solvates). Applying Le Chatelier's principle to the equilibrium between a substance in a crystalline state and its saturated solution
we come to the conclusion that in cases where a substance dissolves with energy absorption, an increase in temperature should lead to an increase in its solubility
In most such cases, with increasing temperature, the mutual solubility of the liquids increases until a temperature is reached at which both liquids are mixed in any proportions.
When solids dissolve in water, the volume of the system usually changes insignificantly. Therefore, the solubility of substances in a solid state is practically independent of pressure.
Liquids can also dissolve in liquids. Some of them are infinitely soluble in one another, that is, they mix with each other in any proportions, such as, for example, alcohol and water, others - mutually dissolve only up to a certain limit.
The temperature at which the limited mutual solubility of liquids becomes unlimited is called the critical dissolution temperature
distribution law, according to which a substance capable of dissolving in two immiscible solvents is distributed between them so that the ratio of its concentrations in these solvents at a constanttemperature remains constant, regardless of the total amount of solute:
C 1 / C 2 = K
Here С 1 and С 2 - the concentration of the solute in the first and second solvents; / (Is the so-called distribution coefficient.
The dissolution of gases in water is an exothermic process. Therefore, the solubility of gases decreases with increasing temperature. If you leave a glass with cold water, then its inner walls are covered with gas bubbles - this is air that was dissolved in water, released from it due to heating. By boiling, you can remove all the air dissolved in it from the water.
However, the dissolution of gases in organic liquids is often accompanied by the absorption of heat; in such cases, as the temperature rises, the gas solubility increases.
Henry's Law: The mass of a gas that dissolves at a constant temperature in a given volume of liquid is directly proportional to the partial pressure of the gas.
Henry's Law can be expressed by the equation
С = kp
where C is the mass concentration of gas in a saturated solution; p is the partial pressure; k - the proportionality coefficient, called Henry's constant (or Henry's coefficient).
We note an important consequence of Henry's law: the volume of a gas dissolving at a constant temperature in a given volume of liquid does not depend on its partial pressure.If there is a mixture of several gases above the liquid, then the solubility of each of them is determined by its partial pressure.
This must be taken into account when calculating the solubility of gases mixed with other gases. Gases obey Henry's law of pi not very high pressures and, moreover, only in the case when they do not enter into chemical interaction with the solvent. At high pressures, when the behavior of all gases differs markedly from ideal, deviations from Henry's law are also observed in the case of gases that do not chemically interact with the solvent.
The solubility of most substances decreases with decreasing temperature, therefore, when hot saturated solutions are cooled, excess solute is usually released. However, if the cooling is carried out carefully and slowly, while protecting the solution from the possibility of particles of the dissolved substance entering it from the outside, then its separation from the solution may not occur. In this case, a solution will be obtained containing significantly more solute than is required for saturation at a given temperature. This phenomenon was discovered and studied in detail by the Russian academician T.E. Lovits (1794), who called such solutions supersaturated. In a calm state, they can remain unchanged for years. But one has only to throw into the solution a crystal of the substance that is dissolved in it, as immediately other crystals begin to grow around it and after a short time the entire excess of the dissolved substance crystallizes. Sometimes crystallization begins from a simple shaking of the solution, as well as from rubbing a glass rod against the walls of the vessel in which the solution is located. During crystallization, a significant amount of heat is released, so that the vessel with the solution heats up noticeably. Form supersaturated solutions very easily Na 2 SO 4 -10 H 2 O (Glauber's salt), Na 2 B 4 0 7 - 10H 2 O (borax), Na 2 S 2 03-5 H 2 0 (sodium thiosulfate).
It follows from what has been said that supersaturated solutions are unstable systems capable of existing only in the absence of solute solids in the system. The possibility of long-term existence of such solutions is explained by the difficulty of the initial appearance of the smallest "seed" crystals, the so-called crystallization centers, from which crystallization spreads to the entire mass of the solution.
the solution is a homogeneous system. Particles of a solute and solvent are in a random thermal movement and are evenly distributed throughout the entire volume of the solution. If you put a concentrated solution of some substance, for example, sugar, into a cylinder, and carefully pour a layer of a more dilute sugar solution on top of it, then at first sugar and water will be unevenly distributed in the volume of the solution.
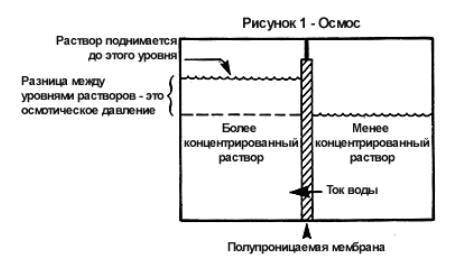 ... However, after a while, the sugar and water molecules will again be evenly distributed throughout the entire volume of the liquid. This is because sugar molecules, randomly moving, penetrate both from a concentrated solution into a diluted one, and in the opposite direction; but at the same time, during any period of time, more sugar molecules pass from a more concentrated solution to a less concentrated solution than from a dilute solution to a concentrated one. In the same way, water molecules move in different directions, but more water molecules pass from a dilute solution, richer in water, to a concentrated solution than during the same time are transferred in the opposite direction. Thus, there is a directed movement of sugar from a concentrated solution to a diluted one, and water - from a dilute solution to a concentrated one; in this case, each substance is transferred to a place where its concentration is lower. Such a spontaneous process of movement of a substance, leading to an equalization of its concentration, is called diffusion.
... However, after a while, the sugar and water molecules will again be evenly distributed throughout the entire volume of the liquid. This is because sugar molecules, randomly moving, penetrate both from a concentrated solution into a diluted one, and in the opposite direction; but at the same time, during any period of time, more sugar molecules pass from a more concentrated solution to a less concentrated solution than from a dilute solution to a concentrated one. In the same way, water molecules move in different directions, but more water molecules pass from a dilute solution, richer in water, to a concentrated solution than during the same time are transferred in the opposite direction. Thus, there is a directed movement of sugar from a concentrated solution to a diluted one, and water - from a dilute solution to a concentrated one; in this case, each substance is transferred to a place where its concentration is lower. Such a spontaneous process of movement of a substance, leading to an equalization of its concentration, is called diffusion.
When measuring the osmotic pressure of various solutions, it was found that the magnitude of the osmotic pressure depends on the concentration of the solution and on its temperature, but does not depend on the nature of the solute or the nature of the solvent. In 1886, Van't Hoff showed that for non-electrolyte solutions of low concentrations, the dependence of osmotic pressure on the concentration of the solution temperature is expressed by the equation (Van't Hoff's law):
P = CRT
Here P is the osmotic pressure of the solution, kPa; C - its molar concentration (molarity), mol / l; R - universal gas constant, 8.314 J / (mol-K); T is the absolute temperature of the solution.
At a given temperature, the saturated vapor pressure above each liquid is a constant value. Experience shows that when a substance is dissolved in a liquid, the saturated vapor pressure of this liquid decreases.
Thus, the pressure of the saturated vapor of the solvent over the solution is always lower than that over the pure solvent at the same temperature. The difference between these values is usually called a decrease in the vapor pressure above the solution (or a decrease in the vapor pressure of a solution). The ratio of the magnitude of this decrease to the pressure of saturated vapor over a pure solution, the body is called the relative decrease in vapor pressure over the solution.
Let us denote the pressure of the saturated vapor of the solvent over the pure solvent through p 0 , and above the solution through the river. Then the relative decrease in vapor pressure over the solution will be a fraction: (Po - P) / Po
In 1887, the French physicist Raoul, studying solutions of various non-volatile liquids and substances in the solid state, established a law relating the decrease in vapor pressure over dilute solutions of non-electrolytes with the concentration:
The relative decrease in the pressure of the saturated vapor of the solvent over the solution is equal to the molar fraction of the solute.
The mathematical expression of Raoult's law is the equation:
(Po - P) / Po = Ni
Here N 2 - the molar fraction of the solute. The phenomenon of a decrease in the saturated vapor pressure over a solution follows from Le Chatelier's principle.
Individual substances are characterized by strictly defined transition temperatures from one state of aggregation to another (boiling point, melting point, sublimation temperature, etc.). So water, at normal atmospheric pressure (101.3 kPa) crystallizes at 0 ° C and boils at 100 ° C.
The situation is different with solutions. The presence of a solute raises the boiling point and lowers the freezing point of the solvent, and the stronger the more concentrated the solution. In most cases, only the solvent crystallizes from the solution from the solution (during freezing) or boils away (during boiling), as a result of which the concentration of the solution increases during its freezing or boiling. This, in turn, leads to an even greater increase in the boiling point and a decrease in the crystallization temperature. Thus, the solution crystallizes and boils not at a certain temperature, but in a certain temperature range. The temperature of the beginning of crystallization and the beginning of boiling of a given solution is called its crystallization temperature and boiling point.
The difference between the boiling points of the solution (t To ) and pure solvent (t OK ) is called an increase in the boiling point of the solution (Δt To ). The difference between the freezing points of a pure solvent (tОЗ) solution (t З ) is called a decrease in the freezing point of the solution (Δt H).
Δt to = t to - t ok; Δt З = t ОЗ - t З.
Any liquid begins to boil at the temperature at which the pressure of its saturated vapor reaches the value of the external pressure. For example, water under a pressure of 101.3 kPa boils at 100 ° C because at this temperature the water vapor pressure is exactly 101.3 kPa. If you dissolve some non-volatile substance in water, then the vapor pressure will decrease. To bring the vapor pressure of the resulting solution to 101.3 kPa, it is necessary to heat the solution above 100 ° C. Hence it follows that the boiling point of the solution is always higher than the boiling point of the pure solvent. The lowering of the freezing point of solutions is explained in a similar way.
Raising the boiling point and lowering the freezing point of solutions correspond to Le Chatelier's principle. Consider the process of freezing a solution. Let there be an equilibrium between liquid and solid phase, for example, water - ice equilibrium at 0 ° С. It can be expressed by the equation:
H 2 O (K) H + + OH -
If you dissolve a certain amount of a substance in water, then the concentration of water molecules in the liquid will decrease and a process will start increasing it - melting of ice. To establish a new equilibrium, it is necessary to lower the temperature.
According to Raoult's second law: for dilute solutions of non-electrolytes, an increase in the boiling point and a decrease in the freezing point are proportional to the concentration of the solution.
ΔT K = E · C m (B); ΔТ З = К · С m (В).
Here С m (B) - molal concentration; E and K - ebulioscopic and cryoscopic constants, depending only on the nature of the solvent, but not depending on the nature of the solute. For water, the cryoscopic constant K is 1.86, the ebulioscopic constant E is 0.52. The ebulioscopic and cryoscopic methods of determination are based on measurements of the boiling and freezing temperatures of solutions. molecular weights substances.
Buffer solutions- solutions, the concentration of hydrogen ions (pH) which does not change from the addition of limited amounts of a strong acid or alkali (see pH). B.R. consist of a mixture of a solution of a weak acid and its salt of a strong base or, conversely, a weak base and its salt of a strong acid, for example: CH 3 COOH + CH 3 СOONa - acetate buffer, NH 4 OH + NHCl - ammonia buffer. Sometimes B.R. can serve as a mixture of solutions of two acidic or acidic and basic salts of a polybasic weak acid and a strong base. For example, phosphate B.p. can be composed of the following pairs: 1) H 3 PO 4 + NaH 2 PO 4; 2) NaH 2 PO 4 + Na 2 HPO 4; 3) Na 2 HPO 4 + Na 3 PO 4 , and carbonate - from 1) H 2 CO 3 + NaHCO 3; 2) NaHCO 3 + Na 2 CO 3 ... B.'s action. is determined by the presence of two interconnected equilibrium systems - dissociation and hydrolysis. To determine the limits of action B. p. the concept of buffer capacity is introduced, which is measured by the amount of a strong acid or base (in g-eq), which must be added to 1 liter of B.R. in order to shift the pH by one. The maximum buffer capacity corresponds to the content of the components in equivalent quantities. In low-mineralized natural waters ah buffering is mainly created by carbonates, i.e. free carbonic acid and its salts of strong bases (Ca, Mg, Na). In sea waters, the borate buffer also participates in the formation of buffering capacity. Buffer capacity of sea water at 0 O C is 11 times higher than that of a NaCl solution with a concentration of 35 OOO and 9 times higher than distilled water. At 30 O The excess is 25 and 19 times, respectively. Such an increase in the buffer capacity of seawater with temperature is associated with an increase in the dissociation and hydrolysis of the components that make up the buffering capacity. Distilled water has a slightly higher buffering capacity than NaCl solution due to better solubility of CO 2 ... Since the pH value does not depend on the concentrations of the components, but on their ratio, then when diluting B.p. it remains constant. At the same time, despite the high buffer capacity of natural waters, the processes of photosynthesis (see) or respiration strongly affect the pH value, since the ratio between the CO concentrations changes. 2 and HCO 3 - ... B.R. play an important role in living organisms. It can be added that strictly fixed pH values in various organs of higher animals and humans, as a rule, are maintained not by one, but by a whole system of biological processes, for example, in the blood - by buffer solutions based on carbonates and phosphates. Acidic or alkaline wastewater entering the reservoir can be neutralized by the carbonate buffer system of natural waters. This also contributes to maintaining the constancy of water pH when adding reagents during water treatment. In biological wastewater treatment (see), the optimal pH values for the normal course of the vital processes of microorganisms are supported by the presence of buffer systems (carbonate, ammonium and phosphate systems). In addition, B.r. widely used in the chemical analysis of water.
Water and solutions Page 8
Aqueous solutions include low-concentrated saturated solutions of inorganic substances, as well as aromatic waters that do not contain alcohol.
Burov's fluid. It is an aqueous solution of basic aluminum acetate with a concentration of 7.6-9.2% and a density of 1.044-1.048. Previously, Burov's liquid was obtained by the reaction of formation of aluminum oxide hydrate with its subsequent dissolution in acetic acid. IN last years it is produced by the electrolysis method developed by A. I. Konovalova. It is based on the process of anodic dissolution of metallic aluminum in an 8% solution of acetic acid when passing through it electric current... As a result, the reaction of formation of basic aluminum acetate proceeds.
Basic lead acetate solution. Lead vinegar. It is an aqueous solution of basic lead acetate with a metallic lead content of 16.7-17.4% and a density of 1.225-1.230. When standing in air, the solution becomes cloudy due to the intense absorption of carbon dioxide from the air. The drug is obtained by the interaction of lead acetate and lead oxide when heated. 3. P. Beridze proposed to obtain a solution of basic lead acetate from lead oxide and acetic acid.
By itself, a basic lead acetate solution is not used. Lead lotion is prepared from it in pharmacies according to the recipe: 2 parts of basic lead acetate and 98 parts of water. Lead lotion, like Burov's fluid, is an astringent and anti-inflammatory agent.
Lime water. Hydroxidation solution. Lime water is a saturated aqueous solution of calcium hydroxide with a concentration of 0.15-0.17%. Received by the reaction of slaking burnt lime (calcium oxide), followed by saturation of the calcium hydroxide solution in the cold. The finished product is a clear, colorless liquid with a strongly alkaline reaction. It is used orally mixed with milk in children's practice with increased acidity of gastric juice and diarrhea.
Potassium arsenite solution. Fowler's arsenic solution. An official solution, which is an aqueous solution of arsenous anhydride (which should be 0.97-1.03% in the preparation) mixed with potassium carbonate.
To obtain a solution of potassium arsenite, 10 parts of potassium carbonate are dissolved in 10 parts of boiling water, 10 parts of arsenous anhydride are added and the liquid is heated to boiling (until complete dissolution). Next, the solution is diluted with 500 g of water and diluted hydrochloric acid is gradually added with stirring until the solution is neutral, which is necessary to prevent the formation of other arsenic salts. After neutralization, 90 parts of alcohol (by volume) and 10 parts of camphor alcohol (by volume) are added to the solution. A solution of potassium arsenite is a list A drug. Camphor alcohol is added to it for the purpose of quick and easy organoleptic identification.
Store the preparation under lock and key (cabinet A), in well-sealed dark glass vials. It is prescribed for anemia, neurasthenia, exhaustion and chronic leukemia.
Fragrant waters. They are weakly concentrated solutions of essential oils in water. These are clear or slightly opalescent liquids with a solute smell. With rare exceptions (dill and bitter almond water), they do not have an independent medicinal purpose and are used as corrective agents (to correct the smell).
Depending on the method of obtaining, simple and distilled aromatic waters are distinguished.
Simple aromatic waters are obtained by direct dissolution of the corresponding essential oil in water in a ratio of 1: 1000 (with the exception of rose water, which is prepared in a ratio of 1: 4000 due to the strong odor of rose oil). Before dissolving, the essential oil is triturated with talc and dissolved in warm (up to 60 °) water. Both operations are necessary to improve the dissolution process. The excess oil in the solution is filtered off through a wet filter.
To increase the stability of simple aromatic waters, it is recommended to add surfactants to them that play the role of solubilizers: tweens, spenes, ethyl stearates and other substances that improve solubility.
Distilled aromatic waters are prepared by distillation, which consists in passing "hot" water vapor through essential oil raw materials. The steam distillation process is based on Dalton's law, according to which two immiscible liquids are distilled at a lower temperature than each separately, since the formation of vapor of such mixtures occurs when the sum of the partial pressures of the mixture components and atmospheric pressure is equal.
To obtain aromatic waters, essential oil raw materials are placed in the distillation cube, through which water vapor is passed, carrying the essential oil along with it into the condenser. In a condenser cooled with cold water, water and essential oil vapors condense and drain into the receiver in the form of finished aromatic water. If during distillation an excess of essential oil is formed that does not dissolve in water, it is poured into separate receivers.
From distilled aromatic waters in pharmaceutical practice, bitter almond water and dill water are more or less widely used, used internally to improve intestinal functions.
Aromatic waters are also prepared with alcohol. In this case, the concentration of essential oil in them can be increased.
Aqueous solutions include low-concentrated saturated solutions of inorganic substances, as well as aromatic waters that do not contain alcohol.
Drilling fluid(Liquor Burovi, Solutio Aluminii subacetatis). It is an aqueous solution of basic aluminum acetate with a concentration of 7.6-9.2% and a density (σ 20) of 1.044-1.048. Previously, Burov's liquid was obtained by the reaction of formation of aluminum oxide hydrate with its subsequent dissolution in acetic acid. In recent years, Burov's liquid has been produced by the electrolysis method developed in 1951 by AI Konovalova. This method is based on the process of anodic dissolution of metallic aluminum in an 8% solution of acetic acid by passing an electric current through it (Fig. 52). As a result, the reaction of formation of basic aluminum acetate proceeds, which in general view can be expressed as follows:
2A l + 2H 2 O + 4CH 3 COOH → 3 H 2 + 2Al (OH) (CH 3 COO) 2
Basic lead acetate solution. Lead vinegar(Solutio Plumbi subacetatis). It is an aqueous solution of basic lead acetate with a metallic lead content of 16.7-17.4% and a density of 1.225-1.230. When standing in air, the solution becomes cloudy due to the intense absorption of carbon dioxide from the air. The drug is obtained by the interaction of lead acetate and lead oxide when heated.
By itself, a basic lead acetate solution is not used. A lead lotion (Aqua Plumbi) is prepared from it in pharmacies according to the recipe: 2 parts of a solution of basic lead acetate and 98 parts of water. Lead lotion, like Burov's fluid, is an astringent and anti-inflammatory agent.
Lime water. Calcium hydroxide solution(Aqua Calcis. Calcium hydroxydum solutum). Lime water is a saturated aqueous solution of calcium hydroxide with a concentration of 0.15-0.17%. Received by the reaction of slaking burnt lime (calcium oxide), followed by saturation of the calcium hydroxide solution in the cold. For this purpose, take 1 part of calcium oxide per 70 parts of freshly boiled distilled water. First, the powder is poured in a small amount (about 1/3) of its amount to obtain calcium hydroxide, and then the rest of the water is insisted on a mushy mass of calcium hydroxide until a saturated aqueous solution is obtained. The finished product is a clear, colorless liquid with a strongly alkaline reaction. It is used orally mixed with milk in pediatric practice with increased acidity of gastric juice and diarrhea.
Potassium arsenite solution. Fowler Arsenic Solution(Liquor Kalii arsenitis. Liquor arsenicalis Fowleri). Official solution (GFH, article No. 378), which is an aqueous solution of arsenous anhydride (which should be 0.97-1.03% in the preparation) mixed with potassium carbonate. The reaction of drug formation is as follows:
As 2 O 3 + K 2 CO 3 1.5H 2 O → 2KAs O 2 + CO 2 + 1.5H 2 O.
To obtain a solution of potassium arsenite, 10 parts of potassium carbonate are dissolved in 10 parts of boiling water, 10 parts of arsenous anhydride are added and the liquid is heated to boiling (until complete dissolution). Next, the solution is diluted with 500 g of water and diluted hydrochloric acid is gradually added with stirring until the solution is neutral, which is necessary to prevent the formation of other arsenic salts. After neutralization, 90 parts of alcohol (by volume) and 10 parts of camphor alcohol (by volume) are added to the solution. A solution of potassium arsenite is a drug of list A. Camphor c pie is added to it for the purpose of quick and easy organoleptic identification.
Store the preparation under lock and key (cabinet A) in well-sealed dark glass vials. It is prescribed for anemia, neurasthenia, exhaustion and chronic leukemia.
Antidote for metal poisoning(Antidotum metallorum). As an antidote for poisoning with heavy metals, a solution of the composition is used: magnesium sulfate crystalline - 3.75 parts, sodium hydrocarbonsite - 12.5 parts, caustic soda (in terms of 100%) - 1 part, hydrogen sulfide - as needed, water - 1000 parts. The technology for preparing the solution is as follows: 500 parts of a 0.2% sodium hydroxide solution are saturated with gaseous hydrogen sulfide, previously passed by bubbling through a suspension of calcium carbonate in water. In another 500 parts of water (freshly prepared and cooled to 50 ° C) sodium bicarbonate and magnesium sulfate are dissolved. The solution is cooled, mixed with the first solution, the combined solutions are cooled to 2-3 ° C below zero and again saturated with hydrogen sulfide until its concentration reaches 0.4%, after which the solution is ready for use. The finished preparation is a lemon-yellow solution with a slightly greenish tint, a pungent smell of hydrogen sulfide and an astringent, salty-bitter taste.
The ions SO 4 2- and S 2- in solution, interacting with heavy metals, convert them into insoluble, non-absorbed compounds in the body, which is the basis for the action of the drug as an antidote.
Fragrant waters(Aquae aromaticae). They are weakly concentrated solutions of essential oils in water. These are clear or slightly dewy liquids with a solute smell. With rare exceptions (dill and bitter almond water), they do not have an independent medicinal purpose and are used as corrective agents (to correct the smell).
Depending on the method of obtaining, simple and distilled aromatic waters are distinguished.
Simple fragrant waters are obtained by direct dissolution of the corresponding essential oil in water in a ratio of 1: 1000 (with the exception of rose water, which is prepared in a ratio of 1: 4000 due to the strong odor of rose oil). Before dissolving, the essential oil is triturated (dispersed) with talc and dissolved in warm (up to 60 ° C) water. Both operations are necessary to improve the dissolution process. The excess oil in the solution is filtered off through a wet filter.
To increase the stability of simple aromatic waters, it is recommended to add surfactants to them that play the role of solubilizers: tweens, spenes, ethyl stearates and other substances that improve solubility.
Distilled aromatic waters prepared by distillation, which consists in passing "hot" water vapor through essential oil raw materials. The steam distillation process is based on Dalton's law, according to which two immiscible liquids are distilled at a lower temperature than each separately, since the formation of vapor of such mixtures occurs when the sum of the partial pressures of the mixture components and atmospheric pressure is equal.
To obtain aromatic waters, essential oil raw materials are placed in the distillation cube, through which water vapor is passed, entraining the essential oil with it into the condenser (Fig. 53).
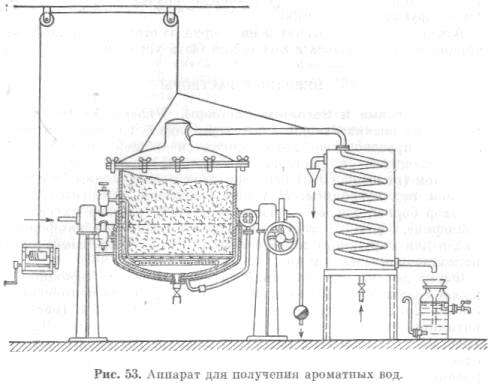
In a condenser cooled with cold water, water and essential oil vapors condense and drain into the receiver in the form of finished aromatic water. If during distillation an excess of essential oil is formed that does not dissolve in water, it is poured into separate receivers.
From distilled aromatic waters in pharmaceutical practice, bitter almond water (Aqua Amygdalarum amararum) and dill water (Aqua Foeniculi), used internally to improve intestinal functions, are more or less widely used.
Aromatic waters are also prepared with alcohol. In this case, the concentration of essential oil in them can be increased.
13.1. Solubility of substances in water
A solution is a homogeneous system consisting of two or more substances, the content of which can be changed within certain limits without disturbing homogeneity.
Aquatic solutions consist of water(solvent) and solute. The state of substances in an aqueous solution, if necessary, is indicated by a subscript (p), for example, KNO 3 in solution - KNO 3 (p).
Solutions that contain a small amount of solute are often called diluted and solutions with a high solute content - concentrated. A solution in which further dissolution of the substance is possible is called unsaturated and the solution in which the substance ceases to dissolve under the given conditions is saturated. The latter solution is always in contact (in heterogeneous equilibrium) with an insoluble substance (one or more crystals).
Under special conditions, for example, when gently (without stirring) cooling a hot unsaturated solution solid substances may form oversaturated solution. When a crystal of a substance is introduced, such a solution is separated into a saturated solution and a precipitate of the substance.
In accordance with chemical theory of solutions D.I.Mendeleev, the dissolution of a substance in water is accompanied, firstly, destruction chemical bonds between molecules (intermolecular bonds in covalent substances) or between ions (in ionic substances), and, thus, particles of a substance mix with water (in which part of the hydrogen bonds between molecules is also destroyed). The breaking of chemical bonds occurs due to the thermal energy of the movement of water molecules, while expenditure energy in the form of heat.
Secondly, once in water, particles (molecules or ions) of the substance are exposed to hydration. As a result, hydrates- compounds of indeterminate composition between particles of matter and water molecules ( internal composition the particles themselves do not change upon dissolution). This process is accompanied by highlighting energy in the form of heat due to the formation of new chemical bonds in hydrates.
In general, the solution is either cools(if the consumption of heat exceeds its release), or heats up (otherwise); sometimes - if the consumption of heat and its release are equal - the temperature of the solution remains unchanged.
Many hydrates are so stable that they are not destroyed even when the solution is completely evaporated. Thus, solid crystalline hydrates of the salts CuSO 4 5H 2 O, Na 2 CO 3 10H 2 O, KAl (SO 4) 2 12H 2 O, etc. are known.
The content of the substance in a saturated solution at T= const quantitatively characterizes solubility of this substance. Typically, solubility is expressed by the mass of the solute per 100 g of water, for example 65.2 g KBr / 100 g H 2 O at 20 ° C. Therefore, if 70 g of solid potassium bromide are introduced into 100 g of water at 20 ° C, then 65.2 g of salt will go into solution (which will be saturated), and 4.8 g of solid KBr (excess) will remain at the bottom of the glass.
It should be remembered that the content of the solute in saturated solution equals, in unsaturated solution less and in oversaturated solution more its solubility at a given temperature. So, a solution prepared at 20 ° C from 100 g of water and sodium sulfate Na 2 SO 4 (solubility 19.2 g / 100 g H 2 O), with a content
15.7 g of salt - unsaturated;
19.2 g salt - saturated;
2O. 3 g of salt - oversaturated.
The solubility of solids (Table 14) usually increases with increasing temperature (KBr, NaCl), and only for some substances (CaSO 4, Li 2 CO 3) the opposite is observed.
The solubility of gases decreases with increasing temperature, and increases with increasing pressure; for example, at a pressure of 1 atm, the solubility of ammonia is 52.6 (20 ° C) and 15.4 g / 100 g H 2 O (80 ° C), and at 20 ° C and 9 atm it is 93.5 g / 100 g H 2 O.
In accordance with the values of solubility, substances are distinguished:
– well soluble whose mass in a saturated solution is comparable to the mass of water (for example, KBr - at 20 ° C solubility is 65.2 g / 100 g H 2 O; 4.6 M solution), they form saturated solutions with a molarity of more than 0.1 M;
– slightly soluble the mass of which in a saturated solution is much less than the mass of water (for example, CaSO 4 - at 20 ° C the solubility is 0.206 g / 100 g H 2 O; 0.015 M solution), they form saturated solutions with a molarity of 0.1-0.001 M;
– practically insoluble whose mass in a saturated solution is negligible compared to the mass of the solvent (for example, AgCl - at 20 ° C the solubility is 0.00019 g per 100 g of H2O; 0.0000134M solution), they form saturated solutions with a molarity of less than 0.001M.
Based on reference data compiled solubility table common acids, bases and salts (Table 15), which indicates the type of solubility, marked substances not known to science (not obtained) or completely decomposed by water.
Conventions used in the table:
"P" is a highly soluble substance
"M" - poorly soluble substance
"N" - practically insoluble substance
"-" - substance not received (does not exist)
"- the substance is mixed with water indefinitely
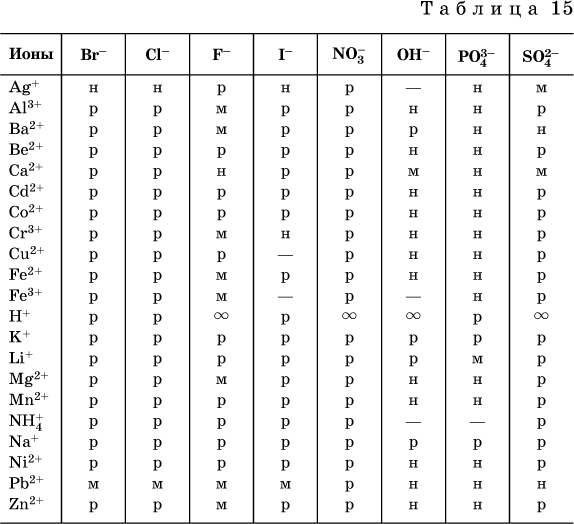
Note. This table corresponds to the preparation of a saturated solution at room temperature by introducing the substance (in the appropriate state of aggregation) into water. It should be noted that it is not always possible to obtain precipitates of poorly soluble substances using ion exchange reactions (for more details see 13.4).




