Malnutrition is one of the prominent and frequent manifestations of chronic obstructive pulmonary disease (COPD), which affects the frequency of exacerbations, respiratory parameters and quality of life of patients. The aim of the study is to assess the nutritional status of COPD patients using anthropometry and bioimpedancemetry methods in a comparative aspect. 60 patients with COPD stages I, II and III were examined. According to the results of the study, a decrease in body mass index (BMI) was found in COPD stages II and III compared with the control group. Loss of the muscle component or lean body mass (TMB) occurs already in COPD stage I, the most significant decrease in TMB was found in stage III of the disease. When comparing the two diagnostic methods, there were no significant differences in BMI and TMT in the general group of COPD patients and at various stages of the disease. When dividing the examined into groups with normal, low and high body mass index, significant differences were found in TMT in the group of patients with BMI >25 kg/m2. In this group, the bioimpedancemetry method has lower TMT values compared to the anthropometry method. Accordingly, the bioelectrical impedance method can be recommended for a more accurate assessment and early diagnosis of protein-energy malnutrition in COPD patients with BMI>25kg/m2.
chronic obstructive pulmonary disease
nutritional deficiencies
anthropometry method
bioimpedancemetry method
1. Avdeev S. N. Chronic obstructive pulmonary disease as a systemic disease // Pulmonology. - 2007. - No. 2.
2. Nevzorova V. A., Barkhatova D. A. Features of the course of COPD exacerbation depending on the nature of the pathogen and the activity of systemic inflammation // Bulletin of Physiology and Pathology of Respiration. - 2006. - No. S 23. - C. 25-30.
3. Nevzorova V. A. Systemic inflammation and the state of the skeletal muscles of patients with COPD / V. A. Nevzorova, D. A. Barkhatova // Therapist. arch. - 2008. - T. 80.
4. Nevzorova V. A. The content of adipokines (leptin and adiponekin) in blood serum in various nutritional status of COPD patients / V. A. Nevzorova, D. A. Barkhatova // Proceedings of the XVIII National Congress on Respiratory Diseases. - Yekaterinburg, 2008.
5. Rudman D. Evaluation of the state of nutrition // Internal diseases. - M.: Medicine, 1993. T. 2.
6. Bernard s., LeBlanc P. et al. Peripheral muscle weakness in patients with chronic obstructive pulmonary desease // Am.J.Respir.Crit.Care. Med. -1998.
7. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI / WHO workshop report. Last updated 2008. www.goldcopd.org/.
8. Body composition by bioelectrica-impedance analysis compared with deuterium dilution and skinfold andthropometry in patients with chronic obstructive pulmonary disease / A.M.W.J.Schols, E.F.M.Wouters,P.B.Soeters et al // Am.J.Clin.Nutr. - 1991.- Vol. 53.- P. 421-424.
9. Prevalence and characteristics of nutritional depletion in patients with stable COPD eligible for pulmonary rehabition / A.M.W.J.Schols, P.B.Soeters, M.C.Dingemans et al // Am.Rev.Respir.Dis. -1993. - Vol. 147. - P. 1151-1156.
IntroductionThe nutritional status reflects the state of the plastic and energy resources of the body, is closely related to the processes of systemic inflammation, oxidative stress, and hormonal imbalance. Malnutrition is one of the most prominent and frequent manifestations of chronic obstructive pulmonary disease (COPD), which affects the frequency of exacerbations, respiratory parameters and quality of life. It has been established that the appearance of protein-energy deficiency aggravates the course of the underlying disease and worsens its prognosis.
Anthropometric measurements are a simple and affordable method that allows using calculation formulas to assess the composition of the patient's body and the dynamics of its change. The ratio of plastic and energy resources can be described through two main components: lean body mass (TMB), which includes muscle, bone and other components and is an indicator of protein metabolism, as well as adipose tissue, indirectly reflecting energy metabolism. With nutritional deficiencies in COPD patients, a disproportionate loss of various components of the body occurs, in which the absence of significant changes in the patient's body weight can mask a protein deficiency while maintaining a normal or somewhat excessive fat component.
The method of anthropometric measurements is not recommended for elderly patients, as well as for edematous syndrome, due to the disproportionate distribution of adipose tissue and its predominant localization in the abdominal cavity. An alternative or more accurate measurement of the composite structure of the body is the bioelectrical impedance method, based on the assessment of the distribution of water volumes, during which the electrical conductivity of tissues is estimated. When conducting impedancemetry, the determination of body composition is based on the greater conductivity of TMT in comparison with body fat, which is associated with different fluid content in these tissues.
Comparison of the information content of widely used methods for assessing nutritional deficiencies in COPD determines the relevance of the study.
Purpose of the study:
To assess the state of the nutritional status of COPD patients using anthropometry and bioimpedancemetry methods in a comparative aspect.
Materials and methods:
We examined 60 patients with phenotypic manifestations of the European race, living in Primorsky Krai for more than 15 years at the age of 63 ± 12.1 years, who were treated in the pulmonology department of City Clinical Hospital No. 1 and the allergorespiratory center in Vladivostok during 2009-2010. with a diagnosis of COPD (general group of patients). All patients were informed about the study in full and filled out informed consent. The control group consisted of 10 healthy non-smoking volunteers, 8 men and 2 women aged 59 ± 10.7 years, who were not relatives of the main group. To diagnose the stage of COPD, the recommendations of the international classification GOLD 2008 were used. All examined patients were divided into 3 groups based on the indicators of the post-bronchodilation test FEV1: group I - 20 patients with stage I COPD (FEV1 = 85 ± 1.3), group II - 20 people with COPD stage II (FEV1=65±1.8), group III - 20 people with COPD stage III (FEV1=40±1.5). The exclusion criteria from the study were the presence of bronchial asthma, myocardial infarction, stroke and other serious diseases, alcohol and drug abuse, elderly people who are not able to understand the goals and objectives of the study, patients' refusal to participate in the study. To assess nutritional deficiencies, methods of anthropometric measurements and calculations of BMI, TMT, as well as bioimpedancemetry and determination of BMI, BFMT (fat-free mass, expressed in %) were used. When calculating the anthropometric parameters of TMT, the Durnin-Womersley (1972) method was used, which is based on the assessment of the average skin-fat fold (SCF) with a caliper, followed by the calculation of TMT according to the formula depending on the gender, age of the patient and BMI. The definition of BMI, which makes it possible to initially diagnose the degree of malnutrition, was determined by the formula of A. Ketele: BMI = MT (kg) / height (m 2).
Bioimpedancemetry was carried out with the help of a reoanalyzer "Diamant" St. Petersburg. The results obtained were processed on an IBM PC running Windows-XP using the Statistica 6.0 program with the calculation of the arithmetic mean (M), its error (± m), and relative error (± m%). Statistical processing when comparing two independent groups was carried out using the nonparametric Mann-Whitney test and the determination of significant differences between groups according to this criterion. Differences between comparative values were recognized as statistically significant at a significance level p<0,05. Анализ взаимосвязей проводился непараметрическим методом корреляционного анализа Спирмена для ненормального распределения с вычислением ошибки коэффициента корреляции.
Research results
The main group of patients had the following anthropometric data: average height 172 ± 5.3 cm, average weight 76.5 ± 5.5 kg. The smoking person index (HCI) averaged 33 ± 2.3, the smoking experience was 30 ± 3.3 years, which indicates a high degree of nicotine-associated risk. We analyzed the ratio of BMI (body mass index) and TMB%, as well as BJMT using anthropometry and bioimpedancemetry methods in COPD patients, depending on the stage of the disease (Table 1).
Table 1. Ratio of BMI, TMT and FBMT in COPD patients
|
Groups surveyed |
Anthropometry method |
Bioimpedancemetry method |
||
|
Indicators |
Indicators |
|||
|
Control group |
||||
|
General group |
25.2±0.4 * |
72.2±1.3 * |
25.0±0.6 * |
71.7±0.7 * |
|
COPD stage I |
75.5±1.1 * |
75.5±0.4 * |
||
|
COPD IIstages |
24.3±0.9 * # |
72.0±1.6 * # |
23.8±0.8* # |
71.65±0.6 # |
|
COPD stage III |
19.9±0.7 * #& |
64.6±1.7 *#& |
19.4±0.5 *#& |
64.2±0.5 *#& |
Note. Significance of differences (p<0,05): * - между группой контроля, общей группой и стадиями ХОБЛ, # - Significance of differences between COPD stages I and II, COPD stages I and III , & - between II and III stages of COPD.
According to the presented results, BMI indices in COPD patients in the general group are lower than in the control group both in the study by anthropometry and bioimpedancemetry. Analysis of BMI values depending on the stage of COPD showed that at stage I of the disease, BMI does not change compared to the control. Its significant decrease occurs only in COPD stages II and III (p<0,05). Несмотря на снижение показателей ИМТ по сравнению с контрольной группой, при всех стадиях ХОБЛ ИМТ находится в пределах референсных значений для нормальных показателей или превышает 20 кг/м 2 . Различий в значениях ИМТ, определенных как методом антропометрии, так и импедансометрии не установлено. Выяснено, что показатели ИМТ при II и III стадиях ХОБЛ достоверно ниже, чем при I стадии ХОБЛ (p<0,05), более того установлено наибольшее снижение показателей ИМТ при III стадии заболевания (p< 0,05).
The data characterizing TMT in the general group of COPD patients, obtained by anthropometry and bioimpedancemetry, are significantly reduced compared to the control group (p<0,05).
The results of the analysis of TMT values depending on the stage of COPD showed that, in contrast to BMI, the loss of TMT occurs already at stage I of COPD. Thus, in COPD stage I, TMT indicators are lower compared to the control (p<0,05). При II и III стадиях ХОБЛ значения ТМТ становятся еще меньше (p<0,05), достигая минимальных результатов при III стадии ХОБЛ (p=0,004). В последнем случае показатели ТМТ достоверно ниже результатов, полученных при исследовании пациентов с I и II стадий ХОБЛ (p<0,05). Во всех группах различий в данных, относящихся к ТМТ, в результате использования методов антропометрии и биоимпедансометрии не установлено.
In contrast to the BMI, which is within the reference interval, for healthy people (BMI 18.5-25 kg / m 2) at all stages of COPD, TMT indicators at stage III of the disease decrease below the recommended values and become below 70%.
Based on the main goal of our study and based on the results of the authors, indicating a greater sensitivity of the bioimpedancemetry method in assessing the nutritional status of patients with signs of obesity and uneven distribution of adipose and muscle tissue, we compared the BMI and TMT in patient groups depending on the mass index body.
To do this, COPD patients were divided into three groups: group I - BMI from 20-25 kg/m 2 , group II - BMI< 20 кг/м 2 и III группа ИМТ >25 kg/m2. The results of the study are presented in table 2.
Table 2. Indicators of MI, TMT, FJMT in COPD patients depending on BMI values
|
Indicator |
Igrueppan=20 |
IIgroupn=20 |
IIIgroupn=20 |
|
BMI20- 25 |
BMI< 2 0 |
BMI>25 |
|
|
TMT (%), anthropometry method |
|||
|
BJMT(%), bioimpedancemetry method |
Note: Significance of differences(p<0,05): *- между ТМТ метода антропометрии и БЖМТ биоимпедансометрии у пациентов ХОБЛ.
As follows from the presented results, significant differences were obtained between the values of TMT as a result of the anthropometry method and BJMT using bioimpedancemetry in COPD patients with BMI>25 kg/m 2 . In this group of patients, TMT indicators were significantly higher than those of FBMT and amounted to 78.5 ± 1.25 and 64.5 ± 1.08 p<0,05 соответственно. Очевидно, что использование метода биоимпедансометрии в группе пациентов ХОБЛ с ИМТ>25kg/m 2 has clear advantages for diagnosing BJMT loss compared to standard anthropometric measurements.
Discussion of the results
COPD is characterized by weight loss associated with protein-energy imbalance. In clinical practice, when determining the nutritional status of patients, it is often limited to calculating only BMI. As a result, it was found that BMI in COPD patients in the general group is lower than in the control group, both in the study by anthropometry and bioimpedancemetry. Analysis of BMI values depending on the stage of COPD showed that at stage I of the disease, BMI does not change compared to the control. Its significant decrease occurs only in COPD stages II and III. At the same time, regardless of the stage of COPD, BMI values are within the reference values for healthy people or exceed 20 kg/m 2 . Accordingly, the definition of BMI is not enough to assess the nutritional status in COPD. To assess body composition, it is necessary to differentiate body fat from lean body mass, since COPD, with a normal or elevated BMI, is characterized by a decrease in muscle mass.
According to our study, TMT values in the general group of COPD patients, assessed by anthropometry and bioimpedancemetry, were significantly reduced compared to the control group (p<0,05). Анализ результатов измерения ТМТ в зависимости от стадии ХОБЛ показал, что в отличие от показателей ИМТ при I стадии заболевания ТМТ достоверно ниже по сравнению с контролем (p<0,05).
In stages II and III of COPD, there is an even more pronounced loss of the protein component of the body weight of patients. This is evidenced by a significant decrease in data characterizing TMT in COPD stages II and III compared with stage I of the disease. The lowest TMT values were found in COPD stage III. Attention is drawn to the fact of the decrease in TMT expressed below the recommended values in stage III COPD. In other words, our study established an advanced loss of TMT in COPD patients compared to BMI. A distinctive feature of our sample is that for all patients with COPD, regardless of stage, BMI is within the recommended values for a healthy population. Despite this, we recorded the fact of a true decrease in TMT in stage III COPD by both methods of research. Taking into account the most pronounced changes in the values of BMI and TMT in COPD stage III, it seemed interesting to us to conduct a correlation analysis between BMI, TMT and FEV1.
The correlation analysis performed showed the absence of significant relationships between FEV1, a diagnostic indicator of the stage of COPD and BMI, in the methods of anthropometry and bioimpedancemetry. At the same time, a direct correlation of the average strength between the values of TMT was established as a result of the study of the anthropometric method and FEV1 (R=0.40+/-0.9; p<0,001) и прямая связь средней силы между данными БЖМТ в результате измерений методом биоимпедансометрии и ОФВ1 (R=0,55+/-0,9; p<0,0005).
Obviously, in COPD, such an indicator of the composite body structure as TMT or BJMT suffers most significantly. Regardless of the presence or absence of signs of hypoxemia, the loss of TMT is directly related to the progression of COPD and a decrease in the rate of respiratory function.
Based on the purpose of the study, the indicators of TMT and BJMT diagnosed using anthropometry and bioimpedancemetry methods do not differ significantly, however, these methods were used with BMI in patients who were not divided into groups with normal, low and high body mass index, which must be taken into account. We analyzed the comparative characteristics of TMT and BJMT as a result of the applied methods for various BMI indicators. Significant differences were revealed between TMT obtained by anthropometry and BJMT, as a result of measurement using the bioimpedansometry method, with BMI> 25 kg/m 2 in COPD patients (p<0,05). Однако при ИМТ (20-25 кг/м 2), находящегося в пределах референсного значения для здоровых людей и при ИМТ<20кг/м 2 , достоверных различий не выявлено.
Obviously, the method of anthropometric measurements is not recommended for patients with BMI>25kg/m 2 due to their predominant concentration of adipose tissue in the abdominal cavity, which leads to an underestimation of the total fat mass.
The method of bioelectrical impedance makes it possible to more accurately determine protein-energy deficiency with a predominant decrease in muscle mass in COPD patients with BMI>25kg/m 2 .
conclusions
- COPD is characterized by the development of nutritional deficiencies, the phenotypic manifestations of which are the loss of lean body mass, which is recorded even with a normal body mass index. There is a loss of lean body mass, the muscle component of the body, already at stage I COPD, the most significant decrease in TMT was found at stage III of the disease (p<0,05).
- In contrast to the body mass index, loss of lean body mass has a direct relationship with the stage of COPD, as evidenced by the correlation analysis performed.
- In the general group of patients, without taking into account body mass indicators, when comparing the methods of anthropometry and bioimpedancemetry, the indicators of BMI and TMT do not differ significantly. The method of bioelectrical impedance makes it possible to more accurately determine protein-energy deficiency with a predominant decrease in muscle mass in COPD patients with BMI>25kg/m 2 .
Reviewers:
- Duizen I. V., Doctor of Medical Sciences, Professor of the Department of General and Clinical Pharmacology of the VSMU, Vladivostok.
- Brodskaya T. A., Doctor of Medical Sciences, Dean of the Faculty of Advanced Studies, VSMU, Vladivostok.
Bibliographic link
Burtseva E.V. STUDY OF THE NUTRITIONAL STATUS OF COPD PATIENTS USING ANTHROPOMETRY AND BIOIMPEDANSOMETRY METHODS // Modern problems of science and education. - 2012. - No. 2.;URL: http://science-education.ru/ru/article/view?id=5912 (date of access: 01.02.2020). We bring to your attention the journals published by the publishing house "Academy of Natural History"
And indeed it is. Preventive medicine is one of the main areas of work of the modern healthcare system. What is its disadvantage? Preventive measures are massive and do not take into account the characteristics of each person. Nowadays, "Preventive Medicine" can be heard more and more often. In Russia, this area is just beginning to develop, and European specialists have been actively developing it for several years. Preventive medicine deals with each person individually, taking into account his characteristics. Thus, the specialist works with each patient according to the system of an individual approach, which significantly increases the effectiveness of preventive measures.
The program for assessing the functional state of the body was developed to study hemostasis (a complex biological process in the body that ensures its viability) in patients over 18 years of age.
At the first stage, you take a blood test to study your nutritional status. It is necessary to comply with the result of the examination, the dietitian will draw up an individual plan for monitoring, correcting the identified violations.
The composition of research within the framework of a comprehensive program:
Nutritional status basic - 3900 rubles.
includes: AST, ALT, GGT, alkaline phosphatase, ferritin, creatinine, urea, uric acid, total protein, albumin, total bilirubin, total cholesterol, triglycerides, HDL-C, LDL-C, CRP, CPK, glycated hemoglobin, ionized calcium, calcium general, sodium, potassium, chlorine, complete blood count, TSH, LDH
Catad_tema Chronic kidney disease - articles
Nutritional status disorders and the importance of a low-protein diet with the use of ketoanalogues of essential amino acids in the prevention of protein-energy malnutrition in patients with chronic kidney disease
Yu.S. Milovanov, I.I. Alexandrova, I.A. Dobrosmyslov First Moscow State Medical University. Sechenov of the Ministry of Health of Russia, Moscow
Target. To determine the possibilities of traditional anthropometry and bioelectrical impedance analysis (BIA) for early diagnosis of nutritional status disorders in CKD patients with glomerulonephritis (GN) at the predialysis stages and regular hemodialysis, to identify the most significant factors of their development and prevention.
Material and methods. The study included 180 patients with GN, including 1BB with chronic GN and 25 with GN with systemic diseases: 13 with systemic lupus erythematosus (SLE) and 12 with various forms of systemic vasculitis. Depending on the diagnosis and stage of CVD, all patients included in the study were randomized into 2 groups. The first group consisted of 155 patients with chronic GN. Group 2 included 25 patients with systemic diseases (SLE, systemic vasculitis). The age of the patients ranged from 21 to 80 years (46.7 ± 10.8 years), there were 61 women and 119 men. ,3 years). TOVP stages are defined according to NKF K/Boe criteria n(2002), with GFR calculated using the ckd epi formula.
Results. Among all 180 patients with ST-UL CVD, nutritional status disorders were detected in 33.9% according to the traditional method and in 34.4% using VID. At the same time, the frequency of nutritional status disorders increased depending on the degree of renal failure. in patients of both groups who received a low-protein diet (MLD) in combination with ketoanalogues of essential amino acids (KD) for at least 12 months before the start of the study (n=39), none of them had nutritional status disorders (VID method) . At the same time, among patients who received MVL, but without the use of keto acids, nutritional status disorders were detected in 1.2% of cases, and among patients who did not restrict protein in the diet (n = 31) - in more than 11% of cases. Among patients of the 1st and 2nd groups who received MVL in combination with keto acids at the predialysis stage for at least 12 months before the start of dialysis treatment, ^ = 39), during the first year of treatment with regular HL, significantly less frequently than among patients (n=61 ), to whom ketoanalogues of essential amino acids were not prescribed in the predialysis period, nutritional status disorders were noted (VID method).
Conclusion. Free CVDs require early diagnosis of nutritional status disorders and regular monitoring, including with the help of VID. The use of ketoanalogues of essential amino acids when using MVL at the pre-dialysis stage of CVP allows maintaining the nutritional status of CVP patients.
Keywords. Epidemiology, nutritional disorders, chronic kidney disease, hemodialysis, low-protein diet, essential amino acid keto analogues
Introduction
One of the urgent problems of nephrology is improving the quality of life and overall "survival" of patients with chronic kidney disease (CKD), the prevalence of which is steadily increasing in the world.
Despite the fact that the introduction of methods of renal replacement therapy (RRT) contributed to an increase in the life expectancy of patients with CKD, a number of new problems have emerged, including those associated with the frequency of nutritional status disorders, protein-energy malnutrition (PEM), especially for patients on regular hemodialysis (GD). Nutritional status disorders have an important prognostic value, since they have a significant impact on the survival and level of rehabilitation of these groups of patients. It was noted that the mortality of patients during the first year of dialysis therapy was 15% among patients with a normal body mass index - an integral indicator of nutritional status, and 39% among patients with a body mass index of less than 19 kg/m 2 .
Currently, anthropometry and bioelectrical impedance analysis (BIA) are simple and accessible non-invasive methods for assessing the degree of nutritional status disorders, including in patients with edema. However, there are no works in which, using anthropometry and bioelectrical impedance analysis, a comparative assessment of the state of the nutritional status of CKD patients at the pre-dialysis stages of CKD and during treatment with regular HD, as well as the study of risk factors for the development of nutritional disorders in these patients, was carried out.
Many studies have shown that limiting the daily protein quota in food to 0.3-0.6 g/kg/day prevents the accumulation of toxic products, reduces or delays the onset of uremic dyspepsia. However, the results of several other studies, including the well-known MDRD study (Modification of Diet in Renal Disease), do not give such a clear conclusion. The difference in the results is explained by the difficulties in the organization of the MBD, its observance, especially on a mass scale, and at the same time in ensuring sufficient calorie content of food (at least 35 kcal/kg/day). How to improve the control of MBD, the compliance of CKD patients, is the subject of ongoing research. The objectives of the study included:
1. Determine the frequency and degree of nutritional status disorders using anthropometry and bioelectrical impedance analysis (BIA).
2. To evaluate the role of a low-protein diet (MBD) in combination with the use of ketoanalogues of essential amino acids in the prevention of nutritional status disorders in patients at the pre-dialysis stage of CKD and subsequently on dialysis.
Material and metopes
The study included 180 patients with GN, among them 155 with chronic and 25 with GN with systemic diseases: 13 with systemic lupus erythematosus (SLE) and 12 with various forms of systemic vasculitis (tab. 1).
Among the 180 patients included in the study, 80 were diagnosed with CKD stages III-IV (ESD of initial and moderate degrees) and among 100 patients - CKD of the UD-stage (ESD - severe - dialysis stage).
Depending on the etiology and stage of CKD, all patients included in the study were randomized into two groups. (tab. 2). The first group consisted of 155 patients with chronic GN, including 22 with stage III CKD (GFR -30 -
59 ml / min / 1.73 m 2), 40 with CKD stage IV (GFR -15-29 ml / min / 1.73 m 2) and 93< 10 мл/мин/1,73 м 2). В группу 2 включены 25 больных с системными заболеваниями: 10 больных ХБП III стадии, 8 - IV и 7 - УД-стадии. Для более точной оценки роли степени почечной недостаточности в развитии нутритивных нарушений больные III стадии обеих групп были разделены на 2 подгруппы: в подгруппу IIIA включены больные с СКФ 45-59 мл/ мин/1,73 м 2 , в ШБ - больных с СКФ 30-44 мл/мин/1,73 м 2 (tab. 2).
| Table 2. Distribution of patients depending on the stage of CKD | ||||
| Patient groups | CKD stage III | CKD stage IV (GFR 15-29 ml / min / 1.73 m 2) | CKD stage VD (GFR< 10 мл/мин/1,73 м 2) |
|
A (GFR 45-59 ml / min / 1.73 m 2) | B (GFR 30-44 ml / min / 1.73 m 2) |
|||
Number of patients |
||||
group 1 (CGN), n = 155 | ||||
group 2 (GN in systemic diseases), n = 25 | ||||
The age of patients ranged from 21 to 80 years (46.7 ± 10.8 years), there were 61 women, 119 men (rice. one). The duration of CKD from the onset of renal dysfunction was 3.5-7.1 years (5.2 ± 1.3 years).
The diagnosis of GN was established according to the clinical picture, 2/3 of the patients the diagnosis was confirmed morphologically during intravital kidney biopsy.
All patients included in group 1 had GN without exacerbation. In 120 patients, a decrease in GFR and an increase in creatinine were combined with a decrease in the size of the kidneys of varying degrees (shrinkage).

Systemic diseases were diagnosed according to the criteria adopted for each nosological form.
In patients of this group, a recurrent course of nephritis was observed, in some patients (10 - SLE, 2 - microscopic polyarteritis, 2 - Wegener's granulomatosis) had a history of exacerbations, clinically proceeding as rapidly progressive nephritis, for the relief of which corticosteroids were treated, including in high doses (pulse therapy). The criterion for inclusion of patients with systemic diseases in the study was the absence of signs of disease activity during the study period (hypocomplementemia, high titer of antibodies to double-stranded DNA, anticytoplasmic antibodies - p- and c-ANCA).
CKD is staged using the NKF K/DOQI (2002) criteria, with GFR calculated using the CKD EPI formula.
In addition to the general clinical examination of patients, taken in the nephrology department, special studies were carried out to solve the tasks. (tab. 3).

To determine the degree of nutritional status disorder in patients with CKD, we used two methods (tab. 3):
|
Table 3 Special research methods |
|
|
Methods for assessing nutritional status disorders |
Study Frequency |
|
Diagnostic methods |
|
|
Traditional: |
|
|
1. Subjective assessment methods (questioning, acquaintance with the anamnesis - identification of characteristic complaints, etiological factors). |
1 time / 3 months |
|
2. Anthropometric: |
1 time / 6 months |
|
3. Laboratory: |
1 time / 3 months |
|
II. Instrumental. |
1 time / 6 months |
|
III. Protein intake and calorie content of food according to three daily food diaries |
1 time / 3 months |
|
IV. Quality of Life Questionnaire SF-36 |
|
2. Instrumental assessment method - determination of the patient's body composition using bioelectrical impedance analysis (BIA monitor, Tanita Company, USA). Anthropometric measurements obtained and results
BIA was supplemented by a subjective general assessment (questioning, acquaintance with the anamnesis - identification of characteristic complaints, etiological factors) and laboratory studies (plasma albumin concentration, absolute number of lymphocytes in peripheral blood, transferrin level in the blood).
When assessing the quality of life, the questionnaire SF-36 (Medical Outcomes Study Survey Short Form-36) in its own modification was used, concerning various aspects of the state of physical and mental health of patients.
When calculating survival, the start of replacement therapy was taken as the end point.
In all 100 patients with CKD Vl-a^mi (eGFR< 10 мл/мин/ 1,73 м 2) использованы стандартный интермиттирующий low-flux-ГД или интермиттирующая гемодиафильтрация (ГДФ) в режиме реального времени (on line).
Statistical processing of the material was carried out using the SPSS 12.0 program. The critical level of significance of the null statistical hypothesis (about the absence of differences and influences) was taken equal to 0.05. For the analysis of qualitative variables, Pearson's x 2 test or Fisher's test for 2 x 2 tables was used. Spearman's two-tailed rank correlation analysis or Pearson's two-tailed correlation analysis was used to determine the strength of the relationship. To identify factors associated with the development of nutritional status disorders, multiple stepwise logistic regression analysis was applied.
results
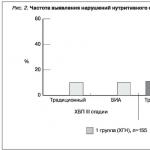
Among all 180 patients with CKD, III-VD stages, nutritional status disorders (PEM) were detected in 33.9% according to the traditional method and in 34.4% using the BIA monitor. At the same time, the frequency of nutritional status disorders depended on the degree of renal insufficiency: among CKD patients with a GFR level of 59-30 ml/min/1.73 m 1%, while among CKD patients with a GFR level of 29–15 ml/min/1.73 m (rice. 2).
Among patients of the 2nd group with CKD in the framework of systemic diseases with high proteinuria (> 1.5 g/day), treatment with corticosteroids in history (> 6 months before inclusion in the study), nutritional disorders were noted even with a moderate decrease in GFR (44-30 ml / min / 1.73 m 2). In the 1st group, they were detected only among patients with stage IV CKD according to both anthropometry and BIA.
Screening results revealed a different number of patients with nutritional status disorders depending on the research method used: traditional - for 59 patients (9% at predialysis stages and 51% at dialysis), and bioimpedance analysis (BIA) - for 64 patients (respectively 10 and 64%. When clarifying the reason for the discrepancy between the results, it turned out that in 5 patients (all women), in whom no nutritional status disorders were detected using the traditional method, moderate edema of the trunk and extremities was noted, which led to an overestimation of the result of anthropometric measurements and the final increase in the calculation of the number of points.
Thus, the BIA method makes it possible to obtain more accurate than the traditional method, which included anthropometric diagnostic parameters, the results of determining the lean and fat mass of patients with edema.
Among the patients of the 1st and 2nd groups observed by us (n = 39), who received MBD in combination with the drug of essential amino acids and their ketoanalogues (EAA and CA) - Ketosteril® for at least 12 months before the start of the study, none of the they did not register violations of the nutritional status (BIA method). At the same time, among patients (n = 10) who received MBD, but without the use of EAC and CA, nutritional status disorders were detected in 1.2%, and among patients (n = 31) who did not restrict protein in the diet, more than 11% of cases (p< 0,05) (tab. 4).
|
Table 4. Frequency of nutritional status disorders among patients with stage III-IV CKD depending on |
|||
|
|
Diets/number of patients (abs. number; %) |
||
|
Patient groups |
MBD (0.6 g/kg/day protein) + essential amino acid keto analogues |
MBD (0.6 g/kg/day protein) |
|
|
group 1 (chronic GN), n = 62 |
|||
|
2nd group (nephritis in systemic diseases), |
|||
|
Total, n = 80 |
|||
|
* The first number of the fraction is the number of patients with impaired nutritional status, the second is the number of patients in the subgroup; % of the total number of patients. |
|||
Using the Pearson pair correlation coefficients (Table 5) the influence on the reduction of body mass index (BMI) as an integral indicator of nutritional status disorders, low caloric intake (< 33 ккал/сут; связь прямая, сильная) (rice. 3), severity of renal failure (GFR< 30 мл/мин/1,73 м 2) (связь прямая, сильная), выраженности анемии (Hb < 9 г/дл; связь прямая, сильная), у больных 2-й группы также высокой протеинурии (>1.5 g/day, feedback, strong) (Fig. 4) and duration of corticosteroid therapy (> 6 months, relationship inverse, strong). The combination of two or more of these factors statistically significantly increased the risk of developing nutritional status disorders.
|
Table 5. Factors affecting the decrease in body mass index (BMI) in patients with stage III-IV CKD (n = 80) 1 |
||||
|
|
Steam coefficient |
Pearson correlations |
||
|
1st group (n = 62) |
2nd group (n = 18) |
1st group (n=62) |
2nd group (n = 18) |
|
|
calorie intake (< 33 ккал/кг/сут) |
||||
|
GFR< 30 (мл/мин/1,73 м 2) |
||||
|
Hb anemia< 9 (г/дл) |
||||
|
Proteinuria > 1.5 (g/day) |
|
|||
|
Treatment with corticosteroids (period > 6 months) |
|
|||

The effect of a low calorie diet on weight loss (by 3-5% per month) is presented in rice. 4. In observed CKD patients at the pre-dialysis stage, persistent proteinuria (> 1.5 g/day) increased the risk of weight loss (rice. 4).

Correspondence of the violation of the nutritional status to the severity of anemia was revealed (the correlation is direct, strong) (rice. 6).

Among patients with CKD stages III-IV of both groups, nutritional status disorders (Table 6) were detected significantly more often among elderly patients (> 65 years), with depressive mood and intolerance to salt-free, unleavened food. These patients often had bacterial and viral infections that aggravated the course of renal failure and nutritional disorders.
In multiple logistic regression modeling, only the presence of a low-calorie diet was significantly and regardless of the development of nutritional disorders (< 33 ккал/кг/сут) (Exp (B) = 6,2 (95 % ДИ - 2,25-16,8; р < 0,001) и СКФ < 30 (мл/мин/1,73 м 2) (Exp (B) = 1,07 (95% ДИ - 1,00-1,13; р = 0,049), у больных 2-й группы также высокой протеинурии (>1.5 g/day) (Exp (B) = 2.05 (95% CI - 1.2-2.5; p = 0.033) and corticosteroid treatment (period > 6 months) (Exp (B) = 2, 01 (95% CI - 1.0-2.13; p = 0.035) when adjusting the model for sex and age.
Among the patients of the 1st and 2nd groups observed by us, who received MBD in combination with EAA and KA preparations at the pre-dialysis stage for at least 12 months before the start of dialysis treatment (^ = 39), nutritional disorders were noted during the first year of treatment with regular HD. status (BIA method) significantly less frequently than among patients (n = 61) who did not receive EAC and CA during the predialysis period (tab. 7). Among patients on program HD in both groups, nutritional status disorders (BIA + laboratory methods were used) were also detected significantly more often, among patients with inadequate dialysis syndrome (Kt/V< 1,0; URR < 65 %), хронического воспаления (инфицированный сосудистый доступ, оппортунистические инфекции, вирусоносительство, гиперпродукция С-реактивного белка), а также при длительном использовании стандартного диализирующего раствора, содержащего уксусную кислоту (tab. 8), and the development of secondary hyperparathyroidism (Fig. 6).
Table 7. The frequency of nutritional status disorders among patients with end-stage CKD during1 the first year of treatment with regular HD depending on the diet used at the pre-dialysis stage (n=100)1 |
|||
Diets in the predialysis period / number of patients (abs. number; %) |
|||
Patient groups | MBD (0.6 g/kg/day of protein) + keto analogs of essential amino acids | MBD (0.6 g/kg/day protein) | No limit on daily protein quota |
group 1 (chronic GN), n = 93 | |||
group 2 (nephritis in systemic diseases), n = 7 | |||
Total (n=100) | |||
* the first number of the fraction is the number of patients with impaired nutritional status, the second is the number of patients in the subgroup; % of the total number of patients |
|||
In 12 patients we observed, dialysis using a concentrate containing acetic acid caused instability of hemodynamic parameters (intradialysis hypotension), nausea, headache, and anorexia. The replacement of all 12 (ash) traditional concentrate for HD with a concentrate that uses hydrochloric acid instead of acetic acid allowed all these patients to exclude intradialytic hypotension and improve the tolerance of HD procedures, normalize appetite.
According to the data given in the literature and the results of our study, an increase in the level of iPTH in the bloodstream enhances catabolism (rapid weight loss against the background of the progression of metabolic acidosis and hyperuricemia), exacerbation of renal failure. An increase in the concentration of iPTH with a deficiency of calcitriol and a decrease in the activity of cellular vitamin D receptors (VDR) in CKD induces the formation of glomerulosclerosis and tubulointerstitial fibrosis.
An inverse correlation was established (r = (-)619; p< 0,01) между ИМТ (кг/м 2) и иПТГ (пг/мл) (rice. 7).

Nutritional status disorders were also detected significantly more often in patients treated with intermittent low-flux HD (x2 = 5.945, p = 0.01), compared with patients treated with intermittent hemodiafiltration (HDF) (tab. 9).
With the help of HDF, due to the high blood flow rate (300-400 ml/min) and intensive ultrafiltration with hemodilution and automatic volumetric control, it was possible to achieve facilitated removal of excess fluid during the procedure, improve nutritional status (normalization of muscle mass and an increase in albumin).
"In patients on program HD, using the Cox regression model, an adverse effect of hypoalbuminemia on the risk of mortality from any cause (cardiovascular complications - CVC, infections, etc.), hospitalizations for CVC, the need to correct the dialysis regimen (for each endpoint separately) was established ) (Fig. 7 and 8).

Compared with patients without hypoalbuminemia among patients with the most severe hypoalbuminemia (< 30 г/л) установлен более высокий риск летальности (отношение шансов - ОШ 1,3; 95% доверительный интервал - ДИ 0,9-1,9), частоты госпитализаций по поводу ССО (ОШ - 2,18; ДИ - 1,76-2,70) и необходимости коррекции режима диализной терапии (ОШ - 5,46; ДИ - 3,38-8,822), причем ОШ отражало изменяющиеся во времени показатели альбумина и Kt/V.
The association of hypoalbuminemia with the studied endpoints became stronger with increasing severity of hypoalbuminemia. Based on these results, the following conclusion can be drawn: the level of albumin decrease is a predictor of poor prognosis and CKD-related complications.
The assessment of the quality of life in groups of patients with identified nutritional status disorders was carried out using a modified form of SF-36. The results of the survey of patients are presented in tab. 10.
According to our data, the prevalence of depression and anxiety, which significantly affect physical activity and social relationships, is 20% in patients with pre-dialysis stages of CKD, and increases to 50% among dialysis patients (p< 0,01). При этом некоторые составляющие качества жизни, такие,как общее самочувствие, утомляемость, склонность к депрессии и тревожность, усугублялись с увеличением диализного стажа.
Discussion and Conclusions
We evaluated the possibilities of determining body composition using the traditional method (which included a subjective assessment of the patient's condition, anthropometric and clinical parameters) in comparison with the BIA method for early diagnosis of nutritional status disorders in CKD patients at the pre-dialysis stages and in dialysis patients.
Table 9. Dynamics of nutritional status in the treatment of HDF (BIA method) |
|||
Indicator | Type of dialysis therapy | ||
Intermittent low-flux HD | Intermittent HDF |
||
BMI, kg / m 2 | |||
Percentage of fat | |||
Percentage of muscle mass | |||
Serum albumin, g/l | |||
Serum transferrin, mg/dl | |||
Among 180 patients, nutritional status disorders were detected in 3.1% of patients with the initial stage of renal failure (CKD stage IIIB) without differences in the frequency of disorders in a comparative assessment by the traditional method of anthropometry and bioelectrical impedance analysis. The incidence of nutritional status disorders increased in direct proportion to the increase in renal failure and depended on the diagnostic method (traditional anthropometry or bioelectrical impedance analysis), amounting to 14.5 and 18.7% for patients with stage IV CKD and 51 and 54% for dialysis patients, respectively.
According to our data, bioelectrical impedance analysis provides more accurate information on the ratio of lean to fat mass of a patient compared to the traditional method of determining the nutritional status, especially in patients with edema. The method is convenient for screening assessment of nutritional status both in the population of patients at the pre-dialysis stage of CKD treatment and in dialysis patients. If anthropometric measurements took an average of 40 ± 10.4 minutes, then the measurement using BIA took 2.5 ± 0.5 minutes.
Diagnosis of the nutritional status using BIA in patients with CKD should also include asking about the patient's complaints, getting acquainted with the anamnesis (identifying characteristic complaints, etiological factors), determining indicators of visceral protein synthesis (the content of albumin in the blood plasma, transferrin and the number of lymphocytes in the peripheral blood).
We assessed the influence of risk factors common to CKD (type of diet, high proteinuria, duration of corticosteroid therapy, depression) and those associated with uremia (secondary hyperparathyroidism, anemia, treatment with program hemodialysis) on the onset or progression of nutritional disorders. It was established in the increase in their frequency and severity of uremia factors and the increase in their role in progressing CKD to Vr-n^rni.
The study showed that more frequent PEI in dialysis patients compared with the pre-dialysis period is caused by a greater severity of depression, anorexia, an additional increase in catabolism on regular HD, as well as the influence of an ineffective dialysis regimen (under-dialysis syndrome).
The use of MBD with the use of ketoanalogues of essential amino acids at the pre-dialysis stage of CKD allows for a rational balanced diet of patients, preventing the development of nutritional status disorders before dialysis, and has a beneficial effect later on dialysis.
In patients with CKD stages III-VD, hypoalbuminemia is closely associated with an increase in concomitant diseases (infections), hospitalizations, and the risk of mortality. Pearson's pairwise correlation coefficients revealed an inverse correlation between the level of acute phase C-reactive protein and albumin in blood serum.
Chronic inflammation syndrome, diagnosed in 18.8% of patients with PEU, was due to the influence of an infected dialysis vascular access and opportunistic infections (pneumonia, urinary infection, etc.). An important role in the induction of chronic inflammation and the development of nutritional disorders was also played by concomitant cardiovascular diseases (CHD, CMP), hypervolemic overhydration, acetate intolerance syndrome, and severe anemia.
The results of our study allow us to expand our understanding of the epidemiology of nutritional disorders in patients with CKD III-VD stages, to identify specific factors that contribute to the development and progression of CKD and PEU in this population. Among patients with CKD, within the framework of systemic diseases, nutritional disorders were already noted with a moderate decrease in GFR (44-30 ml / min / 1.73 m 2), while among patients with chronic GN they were detected with a more pronounced drop in GFR (< 29 мл/мин/1,75 м 2). У всех больных ХБП в период включения в исследование отсутствовали признаки активности заболевания. Однако у подавляющего числа больных системными заболеваниями (СКВ, системные васкулиты), несмотря на развитие ХБП, сохранялась высокая протеинурия (>1.5 g/day) and all of them had a history of exacerbations of the disease, during which patients received long-term (> 6 months) corticosteroids, including ultra-high doses. In patients with CKD in the framework of systemic diseases, a relationship was found between rapid weight loss and high proteinuria (reverse relationship, strong) and the duration of corticosteroid treatment (direct relationship, strong). However, it seems that the role of proteinuria in the development of nutritional disorders was not limited to the loss of protein in the urine. Data have been obtained that proteinuria exceeding 1 g/l, inducing the production of pro-inflammatory cytokines (TNF-a, IL-8) and growth factors (transforming growth factor-p), chemokines (monocyte chemoattractant protein-1, RANTES) by the tubular epithelium and free oxygen radicals, leads to apoptosis of the tubular epithelium with accelerated formation of tubulointerstitial fibrosis and progression of renal failure with a high risk of developing or exacerbating nutritional status disorders. However, the assessment of the role of proteinuria as a leading factor in the progression of CKD (proteinuric remodeling of tubulointerstitium) was not part of the objectives of our study.
The results of our study and the analysis of literature data allowed us to determine the principles of early diagnosis of nutritional status disorders in observed patients with CKD III-VD-stages. (rice. 9).

All patients with CKD receiving a low-protein diet (0.6 g protein/kg/day) with insufficient energy value of food, high proteinuria (> 1.5 g/day), long-term (> 6 months) treatment with corticosteroids.
Screening for protein-energy malnutrition should be performed at the pre-dialysis stage in all individuals with CKD with complaints suggestive of nutritional status disorders:
Progressive weight loss;
depression
aggravation of arterial hypertension, unexplained other causes;
the development of severe anemia that does not correspond to the degree of renal failure (a decrease in erythropoiesis may be due to a decrease in protein synthesis).
Nutritional status should be monitored regularly. Comprehensive assessment of nutritional status in patients with CKD can be quickly carried out using BIA. At the same time, BMI, dry weight dynamics, lean and lean body mass, gastrointestinal symptoms, dialysis time, laboratory data (albumin and blood transferrin), hospitalization rates and the risk of mortality on HD should be analyzed.
The use of ketoanalogues of essential amino acids when using MBD at the predialysis stage of CKD allows maintaining the nutritional status of CKD patients.
Literature
1. Milovanov Yu.S. Nephroprotective strategy in CKD patients at the pre-dialysis stage. Publisher: Lambert Academic Publishing. germany. 2011; 157 p.
2. Nikolaev A.Yu., Milovanov Yu.S. Treatment of renal failure. 2nd edition. M. 2011. Publisher: MIA. 58855 p.
3. KDIGO Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. 2013; (1):3.
4. K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. Am. J. Kidney Dis. 2002;39(suppl 1).
5. Chauveue P., Aparicio A. Benefits in nutritional interventions in patients with CKD stage 3-4. J Renal Nutrition. 2001;21(1):20-22.
6. Milovanov Yu.S. Chronic renal failure. In the book "Rational pharmacotherapy" / ed. ON THE. Mukhina, L.V. Kozlovskaya, E.M. Shilov. Moscow: Litterra. 2006; 13:586-601.
7. Mukhin N.A., Tareeva I.E., Shilov E.M. Diagnosis and treatment of kidney diseases. M.: GEOTAR-MED. 2002; 381 c.
8. Shutov E.V. Nutritional status in patients with chronic renal failure (literature review). Nephrol. dial. 2008; 3-4(10):199-207.
9. Milovanov Yu.S., Nikolaev A.Yu., Lifshits N.L. Diagnosis and principles of treatment of chronic renal failure. Russ. honey. magazine. 1997;23:7-11.
10. Smirnov A.V., Beresneva O.N., Parastaeva M.M. Efficiency of low-protein diets with the use of Ketosteril and soy isolate on the course of experimental renal failure. Nephrol. dial. 2006; 4(8): 344-349.
11. Ermolenko V.M., Kozlova T.A., Mikhailova N.A. The value of a low-protein diet in slowing down the progression of chronic renal failure. Nephrol. and dialysis. 2006; 4:310-320.
12. Kozlovskaya L.V., Milovanov Yu.S. Nutritional status in patients with chronic kidney disease. Nephrology: national guidelines / Ed. ON THE. Mukhin. Moscow: GEOTAR-Media. 2009; 203-210.
13. Kucher A.G., Kayukov I.G., Grigorieva N.D. Medical nutrition at various stages of chronic kidney disease. Nephrol. dial. 2007; 2(9):118-135
14. Milovanov Yu.S. Low-protein diet in chronic kidney disease with renal insufficiency at the pre-dialysis stage: dietary principles. Ter. archive. 2007; 6:39-44.
15. Garneata L., Mircescu G. Keto-analogues in pre-dialysis CKDpatients: review of old and new data. XVI International Congress on Nutrition and Metabolism in Renal Disease 2012, A31.
16. Modification of Diet in Renal Disease (MDRD) Study Group (prepared by Levey AS, Adler S., Caggiula AW, England BK, Grerne T., Hunsicker LG, Kuser JW, Rogers NL, Teschan PE): Effects of dietary protein restriction on moderate renal aisease in the Modification of diet on Renal Disease Study. Am. J. Soc. Nephrol. 1996;7:2616-26.
17. Milovanov Yu.S., Aleksandrova I.I., Milovanova L.Yu. Eating Disorders in Dialysis Treatment of Acute and Chronic Renal Failure, Diagnosis, Treatment (Practical Recommendations). Wedge. nephrol. 2012; 2:22-31.
18. Fouque D. et al. Nutrition and chronic kidney diseases. Kidney International 2011;80:348-357.
Quantification of the patient's nutritional status is an important clinical parameter and should be performed for each patient.
The cost of inpatient treatment of a patient with a normal nutritional status is 1.5-5 times less than that of a patient with malnutrition. In this regard, the most important task of the clinician is to recognize the states of malnutrition and adequate control over their correction. Numerous studies have shown that the state of protein-energy deficiency significantly affects the morbidity and mortality rates among patients.
Obesity and severe malnutrition can be recognized by history and clinical examination, but minor signs of malnutrition are often seen, especially in the presence of edema.
Quantifying nutritional status allows you to detect life-threatening disorders in a timely manner and evaluate positive changes when recovery begins. Objective measures of nutritional status correlate with morbidity and mortality. However, none of the indicators of the quantitative assessment of nutritional status has a clear prognostic significance for a particular patient without taking into account the dynamics of changes in this indicator.
- Nutritional (nutritional, trophological) status of the patient and indications for its assessment
In the domestic literature there is no generally accepted term for assessing the nutrition of the patient. Different authors use the concepts of nutritional status, nutritional status, trophological status, protein-energy status, nutritional status. When assessing the state of nutrition, it is most correct to use the term "nutrition status of the patient", since it reflects both the nutritional and metabolic components of the patient's condition. The ability to timely diagnose malnutrition is necessary in the practice of doctors of all specialties, especially when working with geriatric, gastroenterological, nephrological, endocrine and surgical continents of patients.
Nutritional status should be determined in the following situations:
- In the diagnosis of protein-energy malnutrition.
- When monitoring the treatment of protein-energy malnutrition.
- When predicting the course of the disease and assessing the risk of surgical and unsafe methods of treatment (chemotherapy, radiation therapy, etc.).
- Methods for assessing nutritional status
- Physical examination
Physical examination allows the doctor to diagnose both obesity and protein-energy malnutrition, as well as determine the deficiency of individual nutrients. If a patient is suspected of having a nutrient deficiency, after the examination, it is necessary to confirm the assumption with laboratory tests.
WHO experts describe the following clinical signs of protein-energy malnutrition: protrusion of the bones of the skeleton; loss of skin elasticity; thin, sparse, easily pulled out hair; depigmentation of skin and hair; swelling; muscle weakness; decrease in mental and physical performance.
-
Nutrients Deficiency disorders and symptoms Results of laboratory studies Water Thirst, decreased skin turgor, dry mucous membranes, vascular collapse, mental disorder Increasing the concentration of electrolytes in the blood serum, the osmolarity of the blood serum; a decrease in the total amount of water in the body Calories (energy) Weakness and lack of physical activity, loss of subcutaneous fat, muscle wasting, bradycardia Decreased body weight Protein Psychomotor changes, graying, hair thinning and loss, "scaly" dermatitis, edema, muscle wasting, hepatomegaly, growth retardation Decreased OMP, serum concentrations of albumin, transferrin, protein-bound retinol; anemia; decrease in creatinine / growth, the ratio of urea and creatinine in the urine; increase in the ratio of essential and non-essential amino acids in the blood serum Linoleic acid Xerosis, desquamation, thickening of the stratum corneum, alopecia, fatty liver, delayed wound healing An increase in the ratio of triene and tetraenoic fatty acids in the blood serum Vitamin A Xerosis of eyes and skin, xerophthalmia, Byto's plaque formation, follicular hyperkeratosis, hypogeusia, hyposmia Decrease in the concentration of vitamin A in the blood plasma; increasing the duration of dark adaptation Vitamin D Rickets and growth disorders in children, osteomalacia in adults Increased serum concentration of alkaline phosphatase; a decrease in the concentration of 25-hydroxycholecalciferol in the blood serum Vitamin E Anemia Decrease in the concentration of tocopherol in the blood plasma, hemolysis of erythrocytes Vitamin K Hemorrhagic diathesis Increase in prothrombin time Vitamin C (ascorbic acid) Scurvy, petechiae, ecchymosis, perifollicular hemorrhage, loosening and bleeding gums (or tooth loss) Reducing the concentration of ascorbic acid in the blood plasma, the number of platelets, the mass of whole blood and the number of leukocytes; decrease in the concentration of ascorbic acid in the urine Thiamin (Vitamin B1) Beriberi, muscle soreness and weakness, hyporeflexia, hyperesthesia, tachycardia, cardiomegaly, congestive heart failure, encephalopathy Decreased activity of thiamine pyrophosphate and transketolase contained in erythrocytes and increased in vitro action of thiamine pyrophosphate on it; decrease in the content of thiamine in the urine; increased blood levels of pyruvate and ketoglutarate Riboflavin (vitamin B2) Zaeda (or angular scars), cheilosis, Gunter's glossitis, atrophy of the papillae of the tongue, corneal vascularization, angular blepharitis, seborrhea, scrotal (vulvar) dermatitis Decreased EGR activity and increased effect of flavin adenine dinucleotide on EGR activity in vitro; a decrease in the activity of pyridoxal-phosphate oxidase and an increase in the action of riboflavin on it in vitro; decrease in the concentration of riboflavin in the urine Niacin Pellagra, bright red and "peeled" tongue; atrophy of the papillae of the tongue, fissures of the tongue, pellagrozny dermatitis, diarrhea, dementia Decrease in the content of 1-methyl-nicotinamide and the ratio of 1-methyl-nicotinamide and 2-pyridone in urine
Note: RBMS - basal metabolic rate; BUN, blood urea nitrogen; creatinine/growth - the ratio of the concentration of creatinine in daily urine to growth; ECG - electrocardiogram; EGSUT - erythrocyte glutamine oxaloacetic transaminase; EGR, erythrocyte glutathione reductase; OMP - the circumference of the muscles of the shoulder; KZhST - skin-fat fold over the triceps; RAI - radioactive iodine; Τ, triiodothyronine; Τ, thyroxine; TSH is the pituitary thyroid stimulating hormone.
-
- Anthropometric measurements and body composition analysis
Anthropometric measurements are of particular importance in the physical examination. Anthropometric measurements are a simple and affordable method that allows using calculation formulas to assess the composition of the patient's body and the dynamics of its change. However, when analyzing the data obtained, it must be remembered that tabular data is not always suitable for a particular person. The existing standards were originally calculated for healthy people and may not always be accepted for the patient. It is correct to compare the identified indicators with the data of the same patient in his favorable period.
- Body mass
Determination of body weight (BW) is the baseline for assessing nutritional status.
Body weight is usually compared with the ideal (recommended) body weight. The body weight calculated according to one of the numerous formulas and normograms, or the body weight that was most “comfortable” in the past for this patient, can be taken as the recommended weight.
The edematous syndrome may affect the reliability of the body weight estimate. In the absence of edema, body weight calculated as a percentage of ideal body weight serves as a useful indicator of body fat plus lean body mass. Ideal body weight can be calculated from a standard height/weight table.
With a disproportionate loss of various components of the body, the absence of significant changes in the patient's body weight may mask a protein deficiency while maintaining a normal or slightly excess fat component (for example, the body weight of an emaciated patient who was initially obese may be equal to or exceed the recommended one).
A decrease in the ratio of measured body weight / ideal body weight to 80% or less usually signals an insufficient protein-energy diet.
-
Body weight limits (kg) Height, cm Low Medium High MEN 157,5 58,11-60,84 59,47-64,01 62,65-68,10 160,0 59,02-61,74 60,38-64,92 63,56-69,46 162,6 59,93-62,65 61,29-65,83 64,47-70,82 165,1 60,84-63,56 62,20-67,19 65,38-72,64 167,6 61,74-64,47 63,11-68,55 66,28-74,46 170,2 62,65-65,83 64,47-69,92 67,65-71,73 172,7 63,56-67,19 65,83-71,28 69,01-78,09 175,3 64,47-68,55 67,19-72,64 70,37-79,90 177,8 65,38-69,92 68,55-74,00 71,73-81,72 180,3 66,28-71,28 69,92-75,36 73,09-83,54 182,9 67,65-72,64 71,28-77,18 74,46-85,35 185,4 69,01-74,46 72,64-79,00 76,27-87,17 188,0 70,37-76,27 74,46-80,81 78,09-89,44 190,5 71,73-78,09 75,82-82,63 79,90-91,71 193,04 73,55-79,90 77,63-84,90 82,17-93,98 WOMEN 147,3 46,31-50,39 49,49-54,93 53,57-59,47 149,9 46,76-51,30 50,39-55,84 54,48-60,84 152,4 47,22-52,21 51,30-57,20 55,39-62,20 154,9 48,12-53,57 52,21-58,57 56,75-63,56 157,5 49,03-54,93 53,57-59,93 58,11-64,92 160,0 50,39-56,30 54,93-61,29 59,47-66,74 162,6 51,76-57,66 56,30-62,65 60,84-68,55 165,1 53,12-59,02 57,66-64,01 62,20-70,37 167,6 54,48-60,38 59,02-65,38 63,56-72,19 170,18 55,84-61,74 60,38-66,74 64,92-74,00 172,72 57,20-63,11 61,74-68,10 66,28-75,82 175,26 58,57-64,47 63,11-69,46 67,65-77,18 177,8 59,93-65,83 64,47-70,82 69,01-78,54 180,34 61,29-67,19 65,83-72,19 70,37-79,90 182,88 62,65-68,55 67,19-73,55 71,73-81,27
-
- body composition
Body composition assessment is based on the concept of extracellular and intracellular body mass.
The cell mass is mainly visceral organs and skeletal muscles. The assessment of cell mass is based on the determination of the content of potassium in the body by various, mainly radioisotope methods. The extracellular mass, which performs a predominantly transport function, anatomically includes blood plasma, interstitial fluid, and adipose tissue and is assessed by determining exchangeable sodium. Thus, the intracellular mass reflects mainly the protein component, and the extracellular mass reflects the fat component of the body.
The ratio of plastic and energy resources can be described through two main components: the so-called fat-free or lean body mass (TMB), which includes muscle, bone and other components and is primarily an indicator of protein metabolism, and adipose tissue, which indirectly reflects energy metabolism. .
MT = TMT + fat component.
Thus, to assess body composition, it is sufficient to calculate one of these values. The normal body fat content for men is 15-25%, for women 18-30% of the total body weight, although these figures may vary. Skeletal muscle averages 30% of TMT, the mass of visceral organs is 20%, and bone tissue is 7%.
A decrease in body fat reserves is a sign of a significant deficiency of the energy component of nutrition.
- Methods for determining body composition
To assess the fat content in the body, the method of assessing the average skin-fat fold (anthropometric data) is usually used. There are also various ways to calculate the content of adipose tissue, which are based on determining the density of the human body. Based on the difference in the density of different tissues, the fat component is estimated.
To assess lean body mass, creatinine excretion is studied or bioimpedancemetry is performed.
-
The main method for determining body fat content is based on the assessment of the average skin-fat fold (SKF) with a caliper for several SKFs (most often over the triceps, over the biceps, subscapular and supraileal).
The caliper is a device that allows you to measure the QOL and has a standard fold compression ratio of 10 mg/cm 3 . The manufacture of the caliper is available on an individual basis.
Rules for measuring the skin-fat fold with a caliper.
- Anthropometric measurements are carried out on the non-working (non-dominant) arm and the corresponding half of the body.
- The direction of the folds created during the measurement must coincide with their natural direction.
- Measurements are taken three times, the values are recorded 2 seconds after the device lever is released.
- The skin-fat fold is captured by the researcher with 2 fingers and pulled back by about 1 cm.
- Measurements on the shoulder are carried out with the arm hanging freely along the body.
- Middle of the shoulder: the middle of the distance between the points of articulation of the shoulder with the acromial process of the scapula and the olecranon of the ulna (the circumference of the shoulder is also determined at this level).
- CVJ above the triceps is determined at the level of the middle of the shoulder, above the triceps (in the middle of the back surface of the arm), is located parallel to the longitudinal axis of the limb.
- CVJ above the biceps is determined at the level of the middle of the shoulder, above the triceps (on the front surface of the arm), is located parallel to the longitudinal axis of the limb.
- The subscapular (subscapular) SCJ is defined 2 cm below the angle of the scapula, usually located at an angle of 45° to the horizontal.
- SIJ above the iliac crest (supraileal): determined directly above the iliac crest along the mid-axillary line, usually located horizontally or at a slight angle.
- Anthropometric indicators are determined in the middle third of the shoulder of the non-working arm. Their proportions make it possible to judge the ratio of tissues throughout the body.
- Measurements of the triceps skinfold (TSF) and arm circumference are usually taken, from which the arm muscle circumference (OMC) is calculated.
The calculated values that characterize the masses of the muscles of the shoulder and subcutaneous adipose tissue correlate with a fairly high accuracy, respectively, with lean (OMP) and fat (FAB) body masses, and, accordingly, with the total peripheral reserves of proteins and fat reserves of the body.
On average, anthropometric indicators corresponding to 90-100% of the generally accepted ones are characterized as normal, 80-90% - as corresponding to a mild degree of malnutrition, 70-80% - to a moderate degree, and below 70% - to a severe degree.
Basic anthropometric indicators of nutritional status (according to Heymsfield S.B. et al., 1982)
Indicator Norms men women Skin fold over the triceps (KZhST), mm 12,5 16,5 Shoulder circumference (OP), cm 26 25 Shoulder muscle circumference (OMC), cm
\u003d OP - π × KZhST25,3 23,2 Area of subcutaneous adipose tissue, cm 2
= KZhST×ΟΜΠ/2 – π×KZhST2/417 21 Shoulder muscle area, cm 2
= (ΟΠ – π × KZhST)2/4p51 43 Note: average values are given. Somatometric indicators vary depending on the age group.
Immunological indicators of nutritional status assessment.
- Comprehensive methods for assessing nutritional status
A large number of complex indices and methods have been developed that allow assessing the nutritional status of a patient with varying degrees of reliability. All of them include a combination of anthropometric, biochemical and immunological parameters.
- Decrease in body weight by more than 10%.
- Decreased total blood protein below 65 g/l.
- Decreased blood albumin below 35 g/l.
- Reducing the absolute number of lymphocytes less than 1800 per µl.
Subjective global assessment according to A. S. Detsky et al. (1987) includes a clinical assessment of 5 parameters:
- Weight loss in the last 6 months
- Dietary changes (diet assessment).
- Gastrointestinal symptoms (anorexia, nausea, vomiting, diarrhea) lasting more than 2 weeks.
- Functionality (bed rest or normal physical activity).
- Disease activity (degree of metabolic stress).
In parallel with the above studies, a subjective and physical examination is carried out: loss of subcutaneous fat, muscle wasting, and the presence of edema.
According to the above indicators, patients are divided into three categories:
- With normal nutritional status.
- With moderate exhaustion.
- With severe exhaustion.
The most common is the scoring of 8 diverse markers of nutritional status. Among these indicators, different authors introduce a clinical assessment, anthropometric and biochemical parameters, the results of a skin test with an antigen, etc.
Each of the indicators is evaluated: 3 points - if it is within the normal range, 2 points - if it corresponds to a mild degree of protein-energy deficiency, 1 point - moderate, 0 points - severe. A sum equal to 1–8 points makes it possible to diagnose mild protein-energy malnutrition, 9–16 points – moderate, 17–24 points – severe. A total score of 0 points indicates the absence of malnutrition.
According to the order of the Ministry of Health of the Russian Federation No. 330 of August 5, 2003, the assessment of the state of nutrition is carried out according to indicators, the totality of which characterizes the nutritional status of the patient and his need for nutrients:
- Anthropometric data: height; body mass; body mass index (BMI); shoulder circumference; measurement of the triceps skin-fat fold (TSF).
- Biochemical parameters: total protein; albumen; transferrin.
- Immunological indicators: the total number of lymphocytes.
-
- Methods for determining body composition
- Body mass
- Physical examination
![]() In the last issue, as the base was determined to help us - nutritional science. For a better understanding of the topic, I have to give some concepts and facts from the concept - Nutritional status.
In the last issue, as the base was determined to help us - nutritional science. For a better understanding of the topic, I have to give some concepts and facts from the concept - Nutritional status.
Nutritional Status- is a complex of clinical, anthropometric and laboratory indicators characterizing the quantitative ratio of muscle and fat mass of the patient's body (see Bulletin of Scientific and Technical Development No. 3 (31), 2010)
Recently, facts about a significant increase in people with violations of this nutritional status began to penetrate into the sources of information.
It should be recognized that among the factors predisposing to diseases of internal organs, malnutrition are quite common and significant. Today, this type of malnutrition is significant, such as diet imbalance. Most often, there is a lack of certain amino acids, vitamins, vegetable fats, microelements, dietary fibers in food, while at the same time excessive consumption of cholesterol, animal fats and refined products. Mentioned malnutrition can lead to nutritional insufficiency, changes in the basic functions of internal organs, which contributes to the formation pathology or exacerbation of chronic diseases.
Thus, good nutrition is the basis of the life of the human body and is an important factor in ensuring resistance to pathological processes of various origins.
nutritional status - you need to eat fully
According to the Research Institute of Nutrition of the Russian Academy of Medical Sciences, from 40 to 80% of residents of large cities have impaired immunity, 30% of Russians have various diseases of the digestive system, which sharply worsen the processes of absorption and digestion of food [Medical newspaper, 02/11/2011, “To grow together faster]:
- deficiency of vitamin C in the diet is observed in 70-100% of the population,
- deficiency of B vitamins and folic acid - in 40-80%,
- deficiency of beta-carotene - in 40-60%,
- selenium deficiency - in 85-100%.
A practically healthy body should receive daily 12 vitamins, 20 amino acids, a whole complex of microelements and minerals.
Studies of the Research Institute of Nutrition of the Russian Academy of Medical Sciences have shown that most of the patients admitted to hospitals have significant disorders. nutritional (food) status :
- 20% - exhaustion and malnutrition;
- 50% - disorders of fat metabolism;
- up to 90% have signs of hypo- and beriberi;
- more than 50% have changes in the immune status.
An analysis conducted by the European Association for Clinical Nutrition and Metabolism states trophic insufficiency in patients:
- in surgery in 27-48%;
- in therapy in 46-59%;
- in geriatrics in 26-57%;
- in orthopedics in 39-45%;
- in oncology in 46-88%;
- in pulmonology in 33-63%;
- in gastroenterology in 46-60%;
- among infectious patients in 42-59%;
- with chronic renal failure - 31-59%.
With a slight deficiency of nutrients (proteins, fats, carbohydrates), in cases of illness, compensation mechanisms are activated in the body, which are designed to protect vital organs by redistributing plastic and energy resources:
- cardiac output and myocardial contractility decrease, atrophy and interstitial edema of the heart may develop;
- weakness and atrophy of the respiratory muscles leads to impaired respiratory function and progressive shortness of breath, gastrointestinal damage is manifested by atrophy of the mucous membrane and loss of the villi of the small intestine, leading to malabsorption syndrome;
- the number and functional ability of T-lymphocytes decreases, changes in the properties of B-lymphocytes, granulocytes are noted, which leads to prolonged wound healing;
- the function of the hypothalamic-pituitary system suffers especially.
Here is what was written recently (excerpt)
Attending Doctor #6, 2009
The state of the nutritional status of modern children, the possibility of its correction
N. L. Chernaya, G. V. Melekhova, L. N. Starunova, I. V. Ivanova, N. I. Ryzhova
The data obtained showed that 26% of children had an excess proportion of adipose tissue in the body, and at the same time, only in 10% of children, we found an increase in the subcutaneous fat layer according to caliperometry. A decrease in the thickness of the subcutaneous fat folds was found in 39% of children, and only 11% had a lack of fat. 
Thus, the results obtained indicate a violation of the trophological status in a significantly larger number of examined preschoolers than according to anthropometry data. Not quite comparable results obtained by methods of studying the percentage of fat in the body and caliperometry are due to the fact that the latter characterize the qualitative state of various compartments of the human body. In particular, an increase in the proportion of adipose tissue in a child's body is naturally accompanied by a decrease in the proportion of lean, so-called "lean" mass. Lean (fat-free) body mass consists of skeletal and smooth muscles, mass of visceral organs, cells of the musculoskeletal system. At the same time, lean body mass is subdivided into extracellular mass and cellular mass. With insufficient nutrition at the initial stage, first of all, cell mass is consumed, and in 80% - due to muscles. The decrease in muscle tone, which we found in almost 70% of children, is an indirect confirmation of the suffering of the cellular compartment of the body.
It is known that a decrease in cellular body mass is often accompanied by an increase in extracellular, usually interstitial, fluid. The decrease in tissue turgor revealed in our study in more than 60% of children and the decrease in the thickness of subcutaneous fat folds is evidence of increased hydrophilicity of the body tissues of a modern child (a state of paratrophy).
So it turns out that excess on the shelves is not an indicator of good nutrition
And for a snack
The degree of malnutrition is assessed in accordance with the recommendations of the European Association for Clinical Nutrition and Metabolism (ESPEN).
Regardless of the cause, the clinical consequences of malnutrition are uniform and include the following syndromes:
- astheno-vegetative syndrome;
- muscle weakness, decreased exercise tolerance;
- immunodeficiencies, frequent infections;
- dysbacteriosis (or syndrome of increased contamination of the small intestine);
- polyglandular endocrine insufficiency syndrome;
- fatty degeneration of the liver;
- loss of libido in men, amenorrhea in women;
- polyhypovitaminosis.
It has been established that body weight and mortality are interrelated parameters. It has been proven that mortality increases sharply with a body mass index of less than 19 kg/m2. A body weight deficit of 45–50% is fatal [Russian Medical Journal, 06/29/2011].
------------
Conclusion: Required complete nutrition.