Topic 6. "Electrolysis of solutions and salt melts"
1. Electrolysis is a redox process that occurs on electrodes when an electric current is passed through an electrolyte solution or melt.
2. Cathode - negatively charged electrode. There is a reduction of metal and hydrogen cations (in acids) or water molecules.
3. Anode - a positively charged electrode. Oxidation of the anions of the acid residue and the hydroxyl group (in alkalis) occurs.
4. During the electrolysis of a salt solution, water is present in the reaction mixture. Since water can exhibit both oxidizing and reducing properties, it is a "competitor" for both cathodic and anodic processes.
5. There are electrolysis with inert electrodes (graphite, carbon, platinum) and an active anode (soluble), as well as electrolysis of melts and electrolyte solutions.
CATHODE PROCESSES
If the metal is in a series of voltages:
The position of the metal in a series of stresses
Recovery at the cathode
from Li to Al
Water molecules are reduced: 2H2O + 2e- → H20+ 2OH-
Mn to Pb
Both water molecules and metal cations are restored:
2H2O + 2e- → H20+ 2OH-
Men+ + ne- → Me0
from Cu to Au
Metal cations are reduced: Men+ + ne- → Me0
ANODIC PROCESSES
acid residue
Asm-
Anode
Soluble
(iron, zinc, copper, silver)
Insoluble
(graphite, gold, platinum)
anoxic
Anode metal oxidation
М0 – ne- = Mn+
anode solution
Anion oxidation (except F-)
Acm- - me- = Ac0
Oxygen-containing
Fluoride - ion (F-)
In acidic and neutral environments:
2 H2O - 4e- → O20 + 4H+
In an alkaline environment:
4OH- - 4e- \u003d O20 + 2H2O
Examples of melt electrolysis processes with inert electrodes
In the electrolyte melt, only its ions are present, therefore, electrolyte cations are reduced at the cathode, and anions are oxidized at the anode.
1. Consider the electrolysis of a potassium chloride melt.
Thermal dissociation KCl → K+ + Cl-
K(-) K+ + 1e- → K0
A (+) 2Cl- - 2e- → Cl02
Summary equation:
2KCl → 2K0 + Cl20
2. Consider the electrolysis of a calcium chloride melt.
Thermal dissociation CaCl2 → Ca2+ + 2Cl-
K(-) Ca2+ + 2e- → Ca0
A (+) 2Cl- - 2e- → Cl02
Summary equation:
CaCl2 → Ca0 + Cl20
3. Consider the electrolysis of a melt of potassium hydroxide.
Thermal dissociation of KOH → K+ + OH-
K(-) K+ + 1e- → K0
A (+) 4OH- - 4e- → O20 + 2H2O
Summary equation:
4KOH → 4K0 + O20 + 2H2O
Examples of electrolysis processes of electrolyte solutions with inert electrodes
Unlike melts, in an electrolyte solution, in addition to its ions, there are water molecules. Therefore, when considering the processes on the electrodes, it is necessary to take into account their participation. The electrolysis of a salt solution formed by an active metal, standing in a series of voltages up to aluminum and an acidic residue of an oxygen-containing acid, is reduced to the electrolysis of water. 1. Consider the electrolysis of an aqueous solution of magnesium sulfate. MgSO4 is a salt that is formed by a metal standing in a series of stresses up to aluminum and an oxygen-containing acid residue. Dissociation equation: MgSO4 → Mg2+ + SO42- K (-) 2H2O + 2e- \u003d H20 + 2OH- A (+) 2H2O - 4e- \u003d O20 + 4H + Total equation: 6H2O \u003d 2H20 + 4OH- + O20 + 4H + 2H2O \u003d 2H20 + O20 2. Consider the electrolysis of an aqueous solution of copper (II) sulfate. СuSO4 is a salt formed by a low-active metal and an oxygen-containing acid residue. In this case, electrolysis produces metal, oxygen, and the corresponding acid is formed in the cathode-anode space. Dissociation equation: CuSO4 → Cu2+ + SO42- K (-) Cu2+ + 2e- = Cu0 A (+) 2H2O – 4e- = O20 + 4H+ Summary equation: 2Cu2+ + 2H2O = 2Cu0 + O20 + 4H+ 2CuSO4 + 2H2O = 2Cu0 + О20 + 2Н2SO4
3. Consider the electrolysis of an aqueous solution of calcium chloride. CaCl2 is a salt that is formed by an active metal and an oxygen-free acid residue. In this case, hydrogen, halogen are formed during electrolysis, and alkali is formed in the cathode-anode space. Dissociation equation: CaCl2 → Ca2+ + 2Cl- K (-) 2H2O + 2e- = H20 + 2OH- A (+) 2Cl- – 2e- = Cl20 Summary equation: 2H2O + 2Cl- = Cl20 + 2OH- CaCl2 + 2H2O = Ca (OH)2 + Cl20 + H20 4. Consider the electrolysis of an aqueous solution of copper (II) chloride. CuCl2 is a salt that is formed by a low-active metal and an acidic residue of an oxygen-free acid. In this case, a metal and a halogen are formed. Dissociation equation: CuCl2 → Cu2+ + 2Cl- K (-) Cu2+ + 2e- = Cu0 A (+) 2Cl- – 2e- = Cl20 Summary equation: Cu2+ + 2Cl- = Cu0 + Cl20 CuCl2 = Cu0 + Cl20 5. Consider the process electrolysis of sodium acetate solution. CH3COOHa is a salt formed by the active metal and the acidic residue of a carboxylic acid. Electrolysis produces hydrogen and alkali. Dissociation equation: CH3COONa → CH3COO - + Na+ K (-) 2H2O + 2e- = H20 + 2OH- A (+) 2CH3COO¯- 2e = C2H6 + 2CO2 Summary equation: 2H2O + 2CH3COO¯ = H20 + 2OH - + C2H6 + 2CO2 2Н2О + 2CH3COONa = 2NaОH + Н20 + C2H6 + 2CO2 6. Consider the process of electrolysis of nickel nitrate solution. Ni(NO3)2 is a salt, which is formed by a metal in the range of voltages from Mn to H2 and an oxygen-containing acid residue. In the process, we get metal, hydrogen, oxygen and acid. Dissociation equation: Ni(NO3)2 → Ni2+ + 2NO3- K (-) Ni2+ +2e- = Ni0 2H2O + 2e- = H20 + 2OH- A (+) 2H2O – 4e- = O20 + 4H+ Overall equation: Ni2+ + 2H2O + 2H2O = Ni0 + H20 + 2OH- + O20 + 4H+ Ni(NO3)2 + 2H2O = Ni0 + 2HNO3 + H20 + O20 7. Consider the process of electrolysis of a sulfuric acid solution. Dissociation equation: H2SO4 → 2H+ + SO42- K (-) 2H+ + 2e- = H20 A (+) 2H2O – 4e- = O20 + 4H+ Overall equation: 2H2O + 4H+ = 2H20 + O20 + 4H+ 2H2O = 2H20 + O20
8. Consider the process of electrolysis of sodium hydroxide solution. In this case, only water electrolysis takes place. The electrolysis of solutions of H2SO4, NaNO3, K2SO4, etc. proceeds similarly. Dissociation equation: NaOH → Na+ + OH- K (-) 2H2O + 2e- = H20 + 2OH- A (+) 4OH- – 4e- = O20 + 2H2O Overall equation: 4H2O + 4OH- = 2H20 + 4OH- + O20 + 2H2O 2H2O = 2H20 + O20
Examples of electrolysis processes of electrolyte solutions with soluble electrodes
The soluble anode undergoes oxidation (dissolution) during electrolysis. 1. Consider the process of electrolysis of copper sulfate (II) with a copper anode. During the electrolysis of a solution of copper sulfate with a copper anode, the process is reduced to the release of copper at the cathode and the gradual dissolution of the anode, despite the nature of the anion. The amount of copper sulfate in solution remains unchanged. Dissociation equation: CuSO4 → Cu2+ + SO42- K (-) Cu2+ +2e- → Cu0 A (+) Cu0 - 2e- → Cu2+ transition of copper ions from anode to cathode
Examples of tasks on this topic in the USE options
AT 3. (Var.5)
Establish a correspondence between the formula of a substance and the products of electrolysis of its aqueous solution on inert electrodes.
FORMULA OF THE SUBSTANCE ELECTROLYSIS PRODUCTS
A) Al2(SO4)3 1. metal hydroxide, acid
B) СsOH 2. metal, halogen
C) Hg(NO3)2 3. metal, oxygen
D) AuBr3 4. hydrogen, halogen 5. hydrogen, oxygen 6. metal, acid, oxygen Argument: 1. During the electrolysis of Al2(SO4)3 and СsOH on the cathode, water is reduced to hydrogen. We exclude options 1, 2, 3 and 6. 2. For Al2(SO4)3, water is oxidized to oxygen at the anode. We choose option 5. For CsOH, the hydroxide ion is oxidized to oxygen at the anode. We choose option 5. 3. During the electrolysis of Hg(NO3)2 and АuBr3 on the cathode, metal cations are reduced. 4. For Hg(NO3)2, water is oxidized at the anode. Nitrate ions in solution bind with hydrogen cations, forming nitric acid in the anode space. We choose option 6. 5. For АuBr3, the Br- anion is oxidized at the anode to Br2. We choose option 2.
A
B
V
G
5
5
6
2
AT 3. (Var.1)
Establish a correspondence between the name of the substance and the method of obtaining it.
NAME OF THE SUBSTANCE PRODUCTION BY ELECTROLYSIS A) lithium 1) LiF solution B) fluorine 2) LiF melt C) silver 3) MgCl2 solution D) magnesium 4) AgNO3 solution 5) Ag2O melt 6) MgCl2 melt Argument: 1. Similar to the electrolysis of sodium chloride melt , the process of electrolysis of the lithium fluoride melt proceeds. For options A and B, choose answers 2. 2. Silver can be restored from a solution of its salt - silver nitrate. 3. Magnesium cannot be restored from a salt solution. We choose option 6 - a melt of magnesium chloride.
A
B
V
G
2
2
4
6
AT 3. (Var.9)
Establish a correspondence between the salt formula and the equation of the process occurring on the cathode during the electrolysis of its aqueous solution.
SALT FORMULA EQUATION OF THE CATHODE PROCESS
A) Al(NO3)3 1) 2H2O – 4e- → O2 + 4H+
B) CuCl2 2) 2H2O + 2e- → H2 + 2OH-
C) SbCl3 3) Cu2+ + 1e- → Cu+
D) Cu(NO3)2 4) Sb3+ - 2 e- → Sb5+ 5) Sb3+ + 3e- → Sb0
6) Cu2+ + 2e- → Cu0
The course of reasoning: 1. Processes of reduction of metal or water cations take place on the cathode. Therefore, we immediately exclude options 1 and 4. 2. For Al(NO3)3: the process of water reduction is going on at the cathode. Choose option 2. 3. For CuCl2: Cu2+ metal cations are reduced. Choose option 6. 4. For SbCl3: Sb3+ metal cations are reduced. Select option 5. 5. For Cu(NO3)2: Cu2+ metal cations are reduced. We choose option 6.
A
B
V
G
2
Establish a correspondence between the salt formula and the product formed on an inert anode during the electrolysis of its aqueous solution: for each position indicated by a letter, select the corresponding position indicated by a number.
| SALT FORMULA | PRODUCT ON ANODE | |
| A | B | V | G |
Solution.
In the electrolysis of aqueous solutions of salts, alkalis and acids on an inert anode:
Water is discharged and oxygen is released if it is a salt of an oxygen-containing acid or a salt of hydrofluoric acid;
Hydroxide ions are discharged and oxygen is released if it is alkali;
The acid residue that is part of the salt is discharged, and the corresponding simple substance is released if it is a salt of an oxygen-free acid (except for).
The process of electrolysis of salts of carboxylic acids takes place in a special way.
Answer: 3534.
Answer: 3534
Source: Yandex: USE training work in chemistry. Option 1.
Establish a correspondence between the formula of a substance and the product formed on the cathode during the electrolysis of its aqueous solution: for each position indicated by a letter, select the corresponding position indicated by a number.
| SUBSTANCE FORMULA | ELECTROLYSIS PRODUCT, PRODUCED AT THE CATHODE |
|
Write down the numbers in response, arranging them in the order corresponding to the letters:
| A | B | V | G |
Solution.
During the electrolysis of aqueous solutions of salts, the following is released at the cathode:
Hydrogen, if it is a salt of a metal that is in the series of metal stresses to the left of aluminum;
Metal, if it is a salt of a metal that is in the series of metal voltages to the right of hydrogen;
Metal and hydrogen, if it is a salt of a metal in the series of metal voltages between aluminum and hydrogen.
Answer: 3511.
Answer: 3511
Source: Yandex: USE training work in chemistry. Option 2.
Establish a correspondence between the salt formula and the product formed on an inert anode during the electrolysis of its aqueous solution: for each position indicated by a letter, select the corresponding position indicated by a number.
| SALT FORMULA | PRODUCT ON ANODE | |
Write down the numbers in response, arranging them in the order corresponding to the letters:
| A | B | V | G |
Solution.
During the electrolysis of aqueous solutions of salts of oxygen-containing acids and fluorides, oxygen is oxidized from water, so oxygen is released at the anode. During the electrolysis of aqueous solutions of anoxic acids, the acid residue is oxidized.
Answer: 4436.
Answer: 4436
Establish a correspondence between the formula of a substance and the product that is formed on an inert anode as a result of the electrolysis of an aqueous solution of this substance: for each position indicated by a letter, select the corresponding position indicated by a number.
| SUBSTANCE FORMULA | PRODUCT ON ANODE |
2) sulfur oxide (IV) 3) carbon monoxide (IV) 5) oxygen 6) nitric oxide (IV) |
Write down the numbers in response, arranging them in the order corresponding to the letters:
| A | B | V | G |
What is electrolysis? For a simpler understanding of the answer to this question, let's imagine any source of direct current. For every DC source, you can always find a positive and a negative pole:
Let us connect to it two chemically resistant electrically conductive plates, which we will call electrodes. The plate connected to the positive pole is called the anode, and to the negative pole is called the cathode:
Sodium chloride is an electrolyte; when it melts, it dissociates into sodium cations and chloride ions:
NaCl \u003d Na + + Cl -
It is obvious that the negatively charged chlorine anions will go to the positively charged electrode - the anode, and the positively charged Na + cations will go to the negatively charged electrode - the cathode. As a result of this, both Na + cations and Cl - anions will be discharged, that is, they will become neutral atoms. The discharge occurs through the acquisition of electrons in the case of Na + ions and the loss of electrons in the case of Cl − ions. That is, the process proceeds at the cathode:
Na + + 1e − = Na 0 ,
And on the anode:
Cl − − 1e − = Cl
Since each chlorine atom has an unpaired electron, their single existence is unfavorable and the chlorine atoms combine into a molecule of two chlorine atoms:
Сl∙ + ∙Cl \u003d Cl 2
Thus, in total, the process occurring at the anode is more correctly written as follows:
2Cl - - 2e - = Cl 2
That is, we have:
Cathode: Na + + 1e − = Na 0
Anode: 2Cl - - 2e - = Cl 2
Let's sum up the electronic balance:
Na + + 1e − = Na 0 |∙2
2Cl − − 2e − = Cl 2 |∙1<
Add the left and right sides of both equations half reactions, we get:
2Na + + 2e − + 2Cl − − 2e − = 2Na 0 + Cl 2
We reduce two electrons in the same way as it is done in algebra, we get the ionic equation of electrolysis:
2NaCl (l.) => 2Na + Cl 2
From a theoretical point of view, the case considered above is the simplest, since in the sodium chloride melt, among the positively charged ions, there were only sodium ions, and among the negative ones, only chlorine anions.
In other words, neither Na + cations nor Cl − anions had "competitors" for the cathode and anode.
And what will happen, for example, if instead of a melt of sodium chloride, a current is passed through its aqueous solution? Dissociation of sodium chloride is also observed in this case, but the formation of metallic sodium in an aqueous solution becomes impossible. After all, we know that sodium, a representative of alkali metals, is an extremely active metal that reacts very violently with water. If sodium cannot be reduced under such conditions, then what will be reduced at the cathode?
Let's remember the structure of the water molecule. It is a dipole, that is, it has a negative and a positive pole:
It is due to this property that it is able to “stick around” both the cathode surface and the anode surface:
The following processes may take place:
2H 2 O + 2e - \u003d 2OH - + H 2
2H 2 O - 4e - \u003d O 2 + 4H +
Thus, it turns out that if we consider a solution of any electrolyte, we will see that the cations and anions formed during the dissociation of the electrolyte compete with water molecules for reduction at the cathode and oxidation at the anode.
So what processes will take place at the cathode and at the anode? Discharge of ions formed during the dissociation of the electrolyte or oxidation / reduction of water molecules? Or, perhaps, all of these processes will occur simultaneously?
Depending on the type of electrolyte, a variety of situations are possible during the electrolysis of its aqueous solution. For example, cations of alkali, alkaline earth metals, aluminum and magnesium are simply not able to be reduced in the aquatic environment, since their reduction should have resulted in respectively alkali, alkaline earth metals, aluminum or magnesium, i.e. metals that react with water.
In this case, only the reduction of water molecules at the cathode is possible.
It is possible to remember what process will take place on the cathode during the electrolysis of a solution of any electrolyte, following the following principles:
1) If the electrolyte consists of a metal cation, which in a free state under normal conditions reacts with water, the following process takes place on the cathode:
2H 2 O + 2e - \u003d 2OH - + H 2
This applies to metals that are at the beginning of the Al activity series, inclusive.
2) If the electrolyte consists of a metal cation, which in its free form does not react with water, but reacts with non-oxidizing acids, two processes take place at once, both the reduction of metal cations and water molecules:
Me n+ + ne = Me 0
These metals include those between Al and H in the activity series.
3) If the electrolyte consists of hydrogen cations (acid) or metal cations that do not react with non-oxidizing acids, only electrolyte cations are restored:
2H + + 2e - \u003d H 2 - in the case of acid
Me n + + ne = Me 0 - in the case of salt
At the anode, meanwhile, the situation is as follows:
1) If the electrolyte contains anions of oxygen-free acid residues (except F -), then the process of their oxidation takes place at the anode, water molecules are not oxidized. For instance:
2Cl - - 2e \u003d Cl 2
S 2- − 2e = S o
Fluoride ions are not oxidized at the anode because fluorine is not able to form in an aqueous solution (reacts with water)
2) If the electrolyte contains hydroxide ions (alkalis), they are oxidized instead of water molecules:
4OH - - 4e - \u003d 2H 2 O + O 2
3) If the electrolyte contains an oxygen-containing acid residue (except for organic acid residues) or a fluoride ion (F -) on the anode, the process of oxidizing water molecules takes place:
2H 2 O - 4e - \u003d O 2 + 4H +
4) In the case of an acidic residue of a carboxylic acid on the anode, the following process takes place:
2RCOO - - 2e - \u003d R-R + 2CO 2
Let's practice writing electrolysis equations for various situations:
Example #1
Write the equations for the processes occurring at the cathode and anode during the electrolysis of a zinc chloride melt, as well as the general electrolysis equation.
Solution
When zinc chloride is melted, it dissociates:
ZnCl 2 \u003d Zn 2+ + 2Cl -
Further, attention should be paid to the fact that it is the zinc chloride melt that undergoes electrolysis, and not the aqueous solution. In other words, without options, only the reduction of zinc cations can occur at the cathode, and the oxidation of chloride ions at the anode. no water molecules
Cathode: Zn 2+ + 2e − = Zn 0 |∙1
Anode: 2Cl − − 2e − = Cl 2 |∙1
ZnCl 2 \u003d Zn + Cl 2
Example #2
Write the equations for the processes occurring at the cathode and anode during the electrolysis of an aqueous solution of zinc chloride, as well as the general electrolysis equation.
Since in this case, an aqueous solution is subjected to electrolysis, then, theoretically, water molecules can take part in electrolysis. Since zinc is located in the activity series between Al and H, this means that both the reduction of zinc cations and water molecules will occur at the cathode.
2H 2 O + 2e - \u003d 2OH - + H 2
Zn 2+ + 2e − = Zn 0
The chloride ion is the acidic residue of the oxygen-free acid HCl, therefore, in the competition for oxidation at the anode, chloride ions “win” over water molecules:
2Cl - - 2e - = Cl 2
In this particular case, it is impossible to write the overall electrolysis equation, since the ratio between hydrogen and zinc released at the cathode is unknown.
Example #3
Write the equations for the processes occurring at the cathode and anode during the electrolysis of an aqueous solution of copper nitrate, as well as the general electrolysis equation.
Copper nitrate in solution is in a dissociated state:
Cu(NO 3) 2 \u003d Cu 2+ + 2NO 3 -
Copper is in the activity series to the right of hydrogen, that is, copper cations will be reduced at the cathode:
Cu 2+ + 2e − = Cu 0
Nitrate ion NO 3 - is an oxygen-containing acid residue, which means that in oxidation at the anode, nitrate ions “lose” in competition with water molecules:
2H 2 O - 4e - \u003d O 2 + 4H +
In this way:
Cathode: Cu 2+ + 2e − = Cu 0 |∙2
2Cu 2+ + 2H 2 O = 2Cu 0 + O 2 + 4H +
The equation obtained as a result of addition is the ionic equation of electrolysis. To get the complete molecular electrolysis equation, you need to add 4 nitrate ions to the left and right sides of the resulting ionic equation as counterions. Then we will get:
2Cu(NO 3) 2 + 2H 2 O = 2Cu 0 + O 2 + 4HNO 3
Example #4
Write the equations for the processes occurring at the cathode and anode during the electrolysis of an aqueous solution of potassium acetate, as well as the general electrolysis equation.
Solution:
Potassium acetate in an aqueous solution dissociates into potassium cations and acetate ions:
CH 3 COOK \u003d CH 3 COO − + K +
Potassium is an alkali metal, i.e. is in the electrochemical series of voltages at the very beginning. This means that its cations are not capable of being discharged at the cathode. Instead, water molecules will be restored:
2H 2 O + 2e - \u003d 2OH - + H 2
As mentioned above, the acid residues of carboxylic acids “win” in the competition for oxidation from water molecules at the anode:
2CH 3 COO - - 2e - \u003d CH 3 -CH 3 + 2CO 2
Thus, summing up the electronic balance and adding the two equations of half-reactions at the cathode and anode, we obtain:
Cathode: 2H 2 O + 2e − = 2OH − + H 2 |∙1
Anode: 2CH 3 COO - - 2e - \u003d CH 3 -CH 3 + 2CO 2 | ∙ 1
2H 2 O + 2CH 3 COO - \u003d 2OH - + H 2 + CH 3 -CH 3 + 2CO 2
We have obtained the complete electrolysis equation in ionic form. By adding two potassium ions to the left and right sides of the equation and adding them with counterions, we get the complete electrolysis equation in molecular form:
2H 2 O + 2CH 3 COOK \u003d 2KOH + H 2 + CH 3 -CH 3 + 2CO 2
Example #5
Write the equations for the processes occurring at the cathode and anode during the electrolysis of an aqueous solution of sulfuric acid, as well as the general electrolysis equation.
Sulfuric acid dissociates into hydrogen cations and sulfate ions:
H 2 SO 4 \u003d 2H + + SO 4 2-
Hydrogen cations H + will be reduced at the cathode, and water molecules will be oxidized at the anode, since sulfate ions are oxygen-containing acid residues:
Cathode: 2Н + + 2e − = H 2 |∙2
Anode: 2H 2 O - 4e - = O 2 + 4H + |∙1
4H + + 2H 2 O \u003d 2H 2 + O 2 + 4H +
Reducing the hydrogen ions in the left and right and left sides of the equation, we obtain the equation for the electrolysis of an aqueous solution of sulfuric acid:
2H 2 O \u003d 2H 2 + O 2
As can be seen, the electrolysis of an aqueous solution of sulfuric acid is reduced to the electrolysis of water.
Example #6
Write the equations for the processes occurring at the cathode and anode during the electrolysis of an aqueous solution of sodium hydroxide, as well as the general electrolysis equation.
Dissociation of sodium hydroxide:
NaOH = Na + + OH -
Only water molecules will be reduced at the cathode, since sodium is a highly active metal, and only hydroxide ions at the anode:
Cathode: 2H 2 O + 2e − = 2OH − + H 2 |∙2
Anode: 4OH − − 4e − = O 2 + 2H 2 O |∙1
4H 2 O + 4OH - \u003d 4OH - + 2H 2 + O 2 + 2H 2 O
Let us reduce two water molecules on the left and right and 4 hydroxide ions, and we come to the conclusion that, as in the case of sulfuric acid, the electrolysis of an aqueous solution of sodium hydroxide is reduced to the electrolysis of water.
Electrolysis (Greek elektron - amber + lysis - decomposition) is a chemical reaction that occurs when a direct current passes through an electrolyte. This is the decomposition of substances into their component parts under the influence of an electric current.
The process of electrolysis is the movement of cations (positively charged ions) to the cathode (negatively charged), and negatively charged ions (anions) to the anode (positively charged).
So, anions and cations rush to the anode and cathode, respectively. This is where the chemical reaction takes place. To successfully solve tasks on this topic and write reactions, it is necessary to separate the processes at the cathode and anode. This is how this article will be built.
Cathode
Cations are attracted to the cathode - positively charged ions: Na +, K +, Cu 2+, Fe 3+, Ag +, etc.
To establish what reaction takes place at the cathode, first of all, you need to determine the activity of the metal: its position in the electrochemical series of metal voltages.

If an active metal (Li, Na, K) appears on the cathode, then water molecules are restored instead of it, from which hydrogen is released. If the metal is of medium activity (Cr, Fe, Cd), both hydrogen and the metal itself are released at the cathode. Inactive metals are isolated at the cathode in pure form (Cu, Ag).
I note that aluminum is considered the boundary between active and medium activity metals in a series of voltages. During electrolysis on the cathode, metals up to aluminum (inclusive!) are not restored, instead of them, water molecules are restored - hydrogen is released.
In the event that hydrogen ions - H + are supplied to the cathode (for example, during the electrolysis of acids HCl, H 2 SO 4), hydrogen is reduced from acid molecules: 2H + - 2e \u003d H 2
Anode
Anions are attracted to the anode - negatively charged ions: SO 4 2-, PO 4 3-, Cl -, Br -, I -, F -, S 2-, CH 3 COO -.
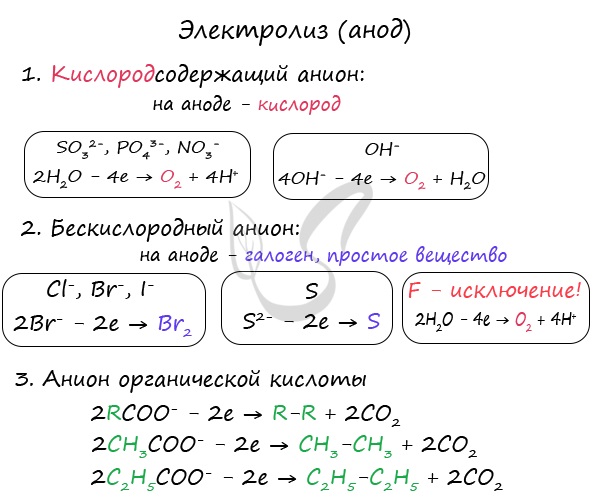
During the electrolysis of oxygen-containing anions: SO 4 2-, PO 4 3- - not anions are oxidized on the anode, but water molecules, from which oxygen is released.
Oxygen-free anions are oxidized and release the corresponding halogens. Sulfide ion in the oxidation of sulfur oxidation. An exception is fluorine - if it hits the anode, then a water molecule is discharged and oxygen is released. Fluorine is the most electronegative element, and therefore is an exception.
Anions of organic acids are oxidized in a special way: the radical adjacent to the carboxyl group is doubled, and the carboxyl group itself (COO) is converted into carbon dioxide - CO 2 .
Solution examples
In the process of training, you may come across metals that are omitted in the activity series. At the training stage, you can use an extended range of metal activity.

Now you will know exactly what is released on the cathode ;-)
So, let's practice. Let us find out what is formed on the cathode and anode during the electrolysis of AgCl, Cu(NO 3) 2 , AlBr 3 , NaF, FeI 2 , CH 3 COOLi solutions.

Sometimes in tasks it is required to record the reaction of electrolysis. I inform you: if you understand what is formed at the cathode and what is at the anode, then writing the reaction is not difficult. Take, for example, the electrolysis of NaCl and write the reaction:
NaCl + H 2 O → H 2 + Cl 2 + NaOH
Sodium is an active metal, so hydrogen is released at the cathode. The anion does not contain oxygen, halogen - chlorine is released. We write the equation so we can't make the sodium evaporate without a trace :) Sodium reacts with water to form NaOH.
Let's write down the electrolysis reaction for CuSO 4:
CuSO 4 + H 2 O → Cu + O 2 + H 2 SO 4
Copper belongs to low-active metals, therefore, in its pure form, it is released at the cathode. The anion is oxygen-containing, so oxygen is released in the reaction. The sulfate ion does not disappear anywhere, it combines with the hydrogen of water and turns into sulfuric acid.
Melt electrolysis
Everything we have discussed up to this point has been about the electrolysis of solutions where the solvent is water.
Industrial chemistry faces an important task - to obtain metals (substances) in a pure form. Inactive metals (Ag, Cu) can be easily obtained by electrolysis of solutions.
But what about active metals: Na, K, Li? After all, during the electrolysis of their solutions, they are not released on the cathode in their pure form, instead of them, water molecules are reduced and hydrogen is released. This is where melts that do not contain water come in handy.

In anhydrous melts, reactions are written even more simply: substances break down into their constituent parts:
AlCl 3 → Al + Cl 2
LiBr → Li + Br2
© Bellevich Yury Sergeevich 2018-2020
This article was written by Yury Sergeevich Bellevich and is his intellectual property. Copying, distribution (including by copying to other sites and resources on the Internet) or any other use of information and objects without the prior consent of the copyright holder is punishable by law. To obtain the materials of the article and permission to use them, please contact
Back forward
Attention! The slide preview is for informational purposes only and may not represent the full extent of the presentation. If you are interested in this work, please download the full version.
The USE results show that tasks on the topic “Electrolysis” remain difficult for graduates. In the school curriculum, an insufficient number of hours are allotted for the study of this topic. Therefore, when preparing students for the exam, it is necessary to study this issue in great detail. Knowledge of the basics of electrochemistry will help the graduate to successfully pass the exam and continue their education in a higher educational institution. To study the topic “Electrolysis” at a sufficient level, it is necessary to carry out preparatory work with graduates passing the exam: - consider the definitions of the basic concepts in the topic “Electrolysis”; - analyze the process of electrolysis melts and solutions of electrolytes; - fix the rules for the reduction of cations at the cathode and the oxidation of anions at the anode (the role of water molecules during the electrolysis of solutions); - the formation of skills to draw up equations for the electrolysis process (cathode and anode processes); - teach students to perform standard tasks of the basic level ( tasks), high and high level of complexity. Electrolysis- redox process occurring in solutions and melts of electrolytes with the passage of a direct electric current. In a solution or melt of an electrolyte, it dissociates into ions. When the electric current is turned on, the ions acquire a directed motion, and redox processes can occur on the surface of the electrodes. Anode- a positive electrode, oxidation processes are taking place on it.
The cathode is a negative electrode, recovery processes are taking place on it.
Melt electrolysis used to obtain active metals located in a series of voltages up to aluminum (inclusive).
Electrolysis of sodium chloride melt
K(-) Na + + 1e -> Na 0
A(+) 2Cl - - 2e -> Cl 2 0
2NaCl (electronic current) -> 2Na + Cl 2 (only for melt electrolysis).
Aluminum is obtained by electrolysis of a solution of aluminum oxide in molten cryolite (Na 3 AlF 6).
2Al 2 O 3 (electronic current) -> 4Al + 3O 2
K(-)Al 3+ +3e‾ ->Al
A(+)2O 2‾ -2e‾ ->O 2
Electrolysis of a melt of potassium hydroxide.
KOH->K + +OH‾
K(-) K + + 1e -> K 0
A (+) 4OH - - 4e -> O 2 0 + 2H 2 O
4KOH (electric current) -> 4K 0 + O 2 0 + 2H 2 O
The electrolysis of aqueous solutions is more difficult, since water molecules can be reduced or oxidized on the electrodes in this case.
Electrolysis of aqueous solutions of salts is more complicated due to the possible participation of water molecules at the cathode and at the anode in the electrode processes.
Rules of electrolysis in aqueous solutions.
On the cathode:
1. Cations located in a series of metal voltages from lithium to aluminum (inclusive), as well as cations NH 4 + are not restored, water molecules are restored instead:
2H 2 O + 2e->H 2 + 2OH -
2. Cations located in the series of voltages after aluminum to hydrogen can be reduced together with water molecules:
2H 2 O + 2e->H 2 + 2OH -
Zn2+ + 2e->Zn 0
3. Cations located in a series of voltages after hydrogen are completely restored: Ag + + 1e->Ag 0
4. Hydrogen ions are reduced in acid solutions: 2H + + 2e->H 2
On the anode:
1. Oxygen-containing anions and F-- do not oxidize, instead of them, water molecules are oxidized:
2H 2 O - 4e->O 2 + 4H +
2.Anions of sulfur, iodine, bromine, chlorine (in this sequence) are oxidized to simple substances:
2Cl - - 2e->Cl 2 0 S 2- - 2e->S0
3. Hydroxide ions are oxidized in alkali solutions:
4OH - - 4e->O 2 + 2H 2 O
4. Anions are oxidized in solutions of salts of carboxylic acids:
2 R - SOO - - 2e->R - R + 2CO 2
5. When using soluble anodes, the anode itself sends electrons to the external circuit due to the oxidation of the atoms of the metal from which the anode is made:
Cu 0 - 2e->Сu 2+
Examples of electrolysis processes in aqueous electrolyte solutions
Example 1 K 2 SO 4 -> 2K + + SO 4 2-
K(-)2H 2 O + 2e‾ -> H 2 + 2OH -
A(+)2H 2 O – 4e‾ -> O 2 + 4H +
The general equation of electrolysis: 2H 2 O (el. current) -> 2 H 2 + O 2
Example 2. NaCl ->Na + +Cl‾
K(-)2H 2 O + 2e‾ -> H 2 + 2OH -
A(+) 2Cl - - 2e -> Cl 2 0
2NaCl + 2H 2 O (el. current) -> H 2 + 2NaOH + Cl 2
Example 3. Cu SO 4 -> Cu 2+ + SO 4 2-
K(-) Cu 2+ + 2e‾ -> Cu
A(+)2H 2 O – 4e‾ -> O 2 + 4H +
General electrolysis equation: 2 Cu SO 4 + 2H 2 O (el. current) -> 2Cu + O 2 + 2H 2 SO 4
Example 4. CH 3 COONa->CH 3 COO‾ +Na +
K(-)2H 2 O + 2e‾ -> H 2 + 2OH -
A(+)2CH 3 COO‾– 2e‾ ->C 2 H 6 +2CO 2
General electrolysis equation:
CH 3 COONa + 2H 2 O (el.current) -> H 2 + 2NaHCO 3 + C 2 H 6
Tasks of the basic level of complexity
Test on the topic “Electrolysis of melts and solutions of salts. A series of stresses of metals”.
1. Alkali is one of the products of electrolysis in an aqueous solution:
1) KCI 2) CuSO 4 3) FeCI 2 4) AgNO 3
2. During the electrolysis of an aqueous solution of potassium nitrate, the following is released at the anode: 1) About 2 2) NO 2 3) N 2 4) H 23. Hydrogen is formed during the electrolysis of an aqueous solution: 1) CaCI 2 2) CuSO 4 3) Hg (NO 3) 2 4) AgNO 34. The reaction is possible between: 1) Ag and K 2 SO 4 (solution) 2) Zn and KCI (solution) 3) Mg and SnCI 2(solution) 4) Ag and CuSO 4 (solution) 5. During the electrolysis of a solution of sodium iodide at the cathode, the color of litmus in solution: 1) red 2 ) blue 3) purple 4) yellow6. During the electrolysis of an aqueous solution of potassium fluoride, the following is released at the cathode: 1) hydrogen 2) hydrogen fluoride 3) fluorine 4) oxygen
Tasks on the topic “Electrolysis”
1. The electrolysis of 400 g of a 20% common salt solution was stopped when 11.2 liters (n.o.) of gas were released at the cathode. The degree of decomposition of the original salt (in%) is:
1) 73 2) 54,8 3) 36,8 4) 18
The solution of the problem. We compose the electrolysis reaction equation: 2NaCl + 2H 2 O → H 2 + Cl 2 + 2NaOHm (NaCl) \u003d 400 ∙ 0.2 \u003d 80 g of salt was in solution. ν (H 2) \u003d 11.2 / 22.4 \u003d 0 .5 mol ν(NaCl)=0.5∙2=1 mol(NaCl)= 1∙58.5=58.5 g of salt was decomposed during electrolysis. Degree of salt decomposition 58.5/80=0.73 or 73%.Answer: 73% of the salt has decomposed.
2. Conducted electrolysis of 200 g of a 10% solution of chromium (III) sulfate until the salt is completely consumed (metal is released on the cathode). The mass (in grams) of water used is:
1) 0,92 2) 1,38 3) 2,76 4) 5,52
The solution of the problem. We compose the electrolysis reaction equation: 2Cr 2 (SO 4) 3 + 6H 2 O → 4Cr + 3O 2 + 6H 2 SO 4m (Cr 2 (SO 4) 3) \u003d 200 ∙ 0.1 \u003d 20g ν (Cr 2 (SO 4) 3) \u003d 20 / 392 \u003d 0.051 mol ν (H 2 O) \u003d 0.051 ∙ 3 \u003d 0.153 mol (H 2 O) \u003d 0.153 18 \u003d 2.76 gTasks of an increased level of complexity B3
1. Establish a correspondence between the salt formula and the equation of the process occurring at the anode during the electrolysis of its aqueous solution.
3. Establish a correspondence between the salt formula and the equation of the process occurring on the cathode during the electrolysis of its aqueous solution.
5. Establish a correspondence between the name of the substance and the electrolysis products of its aqueous solution.
Answers: 1 - 3411, 2 - 3653, 3 - 2353, 4 - 2246, 5 - 145. Thus, studying the topic of electrolysis, graduates master this section well and show good results in the exam. The study of the material is accompanied by a presentation on this topic.